Regulation of XPO5 phosphorylation by PP2A in hepatocellular carcinoma
- PMID: 35441157
- PMCID: PMC9012160
- DOI: 10.1002/mco2.125
Regulation of XPO5 phosphorylation by PP2A in hepatocellular carcinoma
Abstract
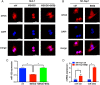
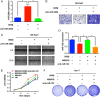
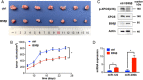
Exportin 5 (XPO5) is a shuttle protein that mediates precursor miRNA (pre-miRNA) export from the nucleus to the cytoplasm, an important step in miRNA maturation. We previously demonstrated that XPO5 was phosphorylated by ERK kinase and subsequently underwent conformation change by the peptidyl-prolyl isomerase Pin1, leading to the reduced miRNA expression in hepatocellular carcinoma (HCC). Protein phosphorylation modification serves as a reversible regulatory mechanism precisely governed by protein kinases and phosphatases. Here we identified that the phosphatase PP2A catalyzed XPO5 dephosphorylation. PP2A holoenzyme is a ternary complex composed of a catalytic subunit, a scaffold subunit, and a regulatory subunit that determines substrate specificity. In this study, we characterized the involvement of B55β subunit in XPO5 dephosphorylation that favored the distribution of XPO5 into the cytoplasm and promoted miRNA expression, leading to HCC inhibition in vitro and in vivo. Our study demonstrates the regulatory role of B55β-containing PP2A in miRNA expression and may shed light on HCC pathogenesis.
Keywords: PP2A; XPO5; hepatocellular carcinoma; microRNA.
© 2022 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The author Yong Peng is an associate editor of MedComm and not involved in the journal's review of, or decisions related to, this manuscript. The authors declare no conflict of interest.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Wong CM, Wong CC, Lee JM, et al. Sequential alterations of microRNA expression in hepatocellular carcinoma development and venous metastasis. Hepatology. 2012;55(5):1453‐1461. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
