This is a preprint.
Famotidine activates the vagus nerve inflammatory reflex to attenuate cytokine storm
- PMID: 35441176
- PMCID: PMC9016653
- DOI: 10.21203/rs.3.rs-1493296/v1
Famotidine activates the vagus nerve inflammatory reflex to attenuate cytokine storm
Update in
-
Famotidine activates the vagus nerve inflammatory reflex to attenuate cytokine storm.Mol Med. 2022 May 16;28(1):57. doi: 10.1186/s10020-022-00483-8. Mol Med. 2022. PMID: 35578169 Free PMC article.
Abstract
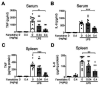
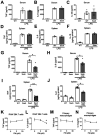
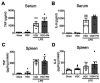
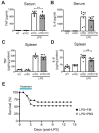
Background. Severe COVID-19 is characterized by pro-inflammatory cytokine release syndrome (cytokine storm) which causes high morbidity and mortality. Recent observational and clinical studies suggest famotidine, a histamine 2 receptor (H2R) antagonist widely used to treat gastroesophageal reflux disease , attenuates the clinical course of COVID-19. Because evidence is lacking for a direct antiviral activity of famotidine, a proposed mechanism of action is blocking the effects of histamine released by mast cells. Here we hypothesized that famotidine activates the inflammatory reflex, a brain-integrated vagus nerve mechanism which inhibits inflammation via alpha 7 nicotinic acetylcholine receptor ( α7nAChR ) signal transduction, to prevent cytokine storm. Methods. The potential anti-inflammatory effects of famotidine and other H2R antagonists was assessed in mice exposed to lipopolysaccharide (LPS)-induced cytokine storm. As the inflammatory reflex is integrated and can be stimulated in the brain, and H2R antagonists penetrate the blood brain barrier poorly, famotidine was administered by intracerebroventricular (ICV) or intraperitoneal (IP) routes. Results. Famotidine administered IP significantly reduced serum and splenic LPS-stimulated tumor necrosis factor α and interleukin-6 concentrations, significantly improving survival. The effects of ICV famotidine were significantly more potent as compared to the peripheral route. Mice lacking mast cells by genetic deletion also responded to famotidine, indicating the anti-inflammatory effects are not mast cell dependent. Either bilateral sub-diaphragmatic vagotomy or genetic knock-out of α7nAChR abolished the anti-inflammatory effects of famotidine, indicating the inflammatory reflex as famotidine's mechanism of action. While the structurally similar H2R antagonist tiotidine displayed equivalent anti-inflammatory activity, the H2R antagonists cimetidine or ranitidine were ineffective even at very high dosages. Conclusions. These observations reveal a previously unidentified vagus nerve-dependent anti-inflammatory effect of famotidine in the setting of cytokine storm which is not replicated by high dosages of other H2R antagonists in clinical use. Because famotidine is more potent when administered intrathecally, these findings are also consistent with a primarily central nervous system mechanism of action.
Conflict of interest statement
Competing interests
HY, SSC and KJT are co-inventors of a patent application (Role of the central nervous nerve system and vagus nerve signaling in famotidine-mediated anti-inflammatory effects). All other authors declare no competing interests.
Figures


References
-
- Moore JB, June CH. (2020) Cytokine release syndrome in severe COVID-19. Science 368: 473–474. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

