Observations and Perspectives on Adaptive Immunity to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
- PMID: 35441229
- PMCID: PMC9383833
- DOI: 10.1093/cid/ciac310
Observations and Perspectives on Adaptive Immunity to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Abstract
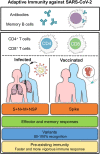
Since the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic began 2 years ago, the scientific community has swiftly worked to understand the transmission, pathogenesis, and immune response of this virus to implement public health policies and ultimately project an end to the pandemic. In this perspective, we present our work identifying SARS-CoV-2 epitopes to quantify T-cell responses and review how T cells may help protect against severe disease. We examine our prior studies which demonstrate durable humoral and cell-mediated memory in natural infection and vaccination. We discuss how SARS-CoV-2-specific T cells from either natural infection or vaccination can recognize emerging variants of concern, suggesting that the currently approved vaccines may be sufficient. We also discuss how pre-existing cross-reactive T cells promote rapid development of immune memory to SARS-CoV-2. We finally posit how identifying SARS-CoV-2 epitopes can help us develop a pan-coronavirus vaccine to prepare for future pandemics.
Keywords: epitopes; immune memory; pre-existing cross-reactive; variants.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. D. W.’s and S. C.’s affiliated institution, La Jolla Institute for Immunology, has filed for patent protection for various aspects of T-cell epitome and vaccine design work. S. C. has received payments or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or education events with JP Morgan, Citi, Morgan Stanley, Avalia NZ, J&J, and Roche. They have also received payment for expert testimony from the University of California, California State University, the State of California, and United Airlines. They have also participated in a data safety monitoring or advisory board with GSK. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous