Cortical phase-amplitude coupling is key to the occurrence and treatment of freezing of gait
- PMID: 35441231
- PMCID: PMC9337810
- DOI: 10.1093/brain/awac121
Cortical phase-amplitude coupling is key to the occurrence and treatment of freezing of gait
Abstract
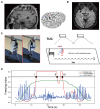
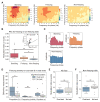
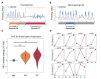
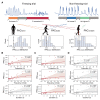
Freezing of gait is a debilitating symptom in advanced Parkinson's disease and responds heterogeneously to treatments such as deep brain stimulation. Recent studies indicated that cortical dysfunction is involved in the development of freezing, while evidence depicting the specific role of the primary motor cortex in the multi-circuit pathology of freezing is lacking. Since abnormal beta-gamma phase-amplitude coupling recorded from the primary motor cortex in patients with Parkinson's disease indicates parkinsonian state and responses to therapeutic deep brain stimulation, we hypothesized this metric might reveal unique information on understanding and improving therapy for freezing of gait. Here, we directly recorded potentials in the primary motor cortex using subdural electrocorticography and synchronously captured gait freezing using optoelectronic motion-tracking systems in 16 freely-walking patients with Parkinson's disease who received subthalamic nucleus deep brain stimulation surgery. Overall, we recorded 451 timed up-and-go walking trials and quantified 7073 s of stable walking and 3384 s of gait freezing in conditions of on/off-stimulation and with/without dual-tasking. We found that (i) high beta-gamma phase-amplitude coupling in the primary motor cortex was detected in freezing trials (i.e. walking trials that contained freezing), but not non-freezing trials, and the high coupling in freezing trials was not caused by dual-tasking or the lack of movement; (ii) non-freezing episodes within freezing trials also demonstrated abnormally high couplings, which predicted freezing severity; (iii) deep brain stimulation of subthalamic nucleus reduced these abnormal couplings and simultaneously improved freezing; and (iv) in trials that were at similar coupling levels, stimulation trials still demonstrated lower freezing severity than no-stimulation trials. These findings suggest that elevated phase-amplitude coupling in the primary motor cortex indicates higher probabilities of freezing. Therapeutic deep brain stimulation alleviates freezing by both decoupling cortical oscillations and enhancing cortical resistance to abnormal coupling. We formalized these findings to a novel 'bandwidth model,' which specifies the role of cortical dysfunction, cognitive burden and therapeutic stimulation on the emergence of freezing. By targeting key elements in the model, we may develop next-generation deep brain stimulation approaches for freezing of gait.
Keywords: Parkinson’s disease; deep brain stimulation; freezing of gait; motor cortex; phase amplitude coupling.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


Comment in
-
Towards adaptive deep brain stimulation for freezing of gait.Brain. 2022 Jul 29;145(7):2236-2238. doi: 10.1093/brain/awac172. Brain. 2022. PMID: 35848860 Free PMC article.
References
-
- Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov Disord. 2007;22(15):2192–2195. - PubMed
-
- Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ; CARPA Study Group . Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70(23):2241–2247. - PubMed
-
- Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol. 2012;11(5):429–442. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

