Development of a Spray-Dried Formulation of Peptide-DNA Nanoparticles into a Dry Powder for Pulmonary Delivery Using Factorial Design
- PMID: 35441318
- PMCID: PMC9197895
- DOI: 10.1007/s11095-022-03256-4
Development of a Spray-Dried Formulation of Peptide-DNA Nanoparticles into a Dry Powder for Pulmonary Delivery Using Factorial Design
Abstract
Background: Gene therapy via pulmonary delivery holds the potential to treat various lung pathologies. To date, spray drying has been the most promising method to produce inhalable powders. The present study determined the parameters required to spray dry nanoparticles (NPs) that contain the delivery peptide, termed RALA (N-WEARLARALARALARHLARALARALRACEA-C), complexed with plasmid DNA into a dry powder form designed for inhalation.
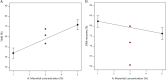
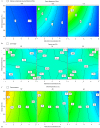
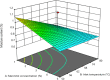
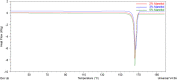
Methods: The spray drying process was optimised using full factorial design with 19 randomly ordered experiments based on the combination of four parameters and three centre points per block. Specifically, mannitol concentration, inlet temperature, spray rate, and spray frequency were varied to observe their effects on process yield, moisture content, a median of particle size distribution, Z-average, zeta potential, encapsulation efficiency of DNA NPs, and DNA recovery. The impact of mannitol concentration was also examined on the spray-dried NPs and evaluated via biological functionality in vitro.
Results: The results demonstrated that mannitol concentration was the strongest variable impacting all responses apart from encapsulation efficiency. All measured responses demonstrated a strong dependency on the experimental variables. Furthermore, spray drying with the optimal variables in combination with a low mannitol concentration (1% and 3%, w/v) produced functional RALA/pDNA NPs.
Conclusion: The optimal parameters have been determined to spray dry RALA/pDNA NPs into an dry powder with excellent biological functionality, which have the potential to be used for gene therapy applications via pulmonary delivery.
Keywords: cell-penetrating peptide; dry powder formulation; gene delivery; pulmonary delivery; spray drying.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


Similar articles
-
Shaping the future from the small scale: dry powder inhalation of CRISPR-Cas9 lipid nanoparticles for the treatment of lung diseases.Expert Opin Drug Deliv. 2023 Apr;20(4):471-487. doi: 10.1080/17425247.2023.2185220. Epub 2023 Mar 12. Expert Opin Drug Deliv. 2023. PMID: 36896650 Free PMC article. Review.
-
Design, characterization, and aerosol dispersion performance modeling of advanced co-spray dried antibiotics with mannitol as respirable microparticles/nanoparticles for targeted pulmonary delivery as dry powder inhalers.J Pharm Sci. 2014 Sep;103(9):2937-2949. doi: 10.1002/jps.23955. Epub 2014 Apr 16. J Pharm Sci. 2014. PMID: 24740732
-
Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery.Drug Des Devel Ther. 2013 Aug 28;7:861-73. doi: 10.2147/DDDT.S47681. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 24039397 Free PMC article.
-
Design, characterization, and aerosol dispersion performance modeling of advanced spray-dried microparticulate/nanoparticulate mannitol powders for targeted pulmonary delivery as dry powder inhalers.J Aerosol Med Pulm Drug Deliv. 2014 Apr;27(2):81-93. doi: 10.1089/jamp.2013.1078. Epub 2014 Feb 6. J Aerosol Med Pulm Drug Deliv. 2014. PMID: 24502451 Free PMC article.
-
Spray-Dried Inhalable Powder Formulations of Therapeutic Proteins and Peptides.AAPS PharmSciTech. 2021 Jun 18;22(5):185. doi: 10.1208/s12249-021-02043-5. AAPS PharmSciTech. 2021. PMID: 34143327 Review.
Cited by
-
Shaping the future from the small scale: dry powder inhalation of CRISPR-Cas9 lipid nanoparticles for the treatment of lung diseases.Expert Opin Drug Deliv. 2023 Apr;20(4):471-487. doi: 10.1080/17425247.2023.2185220. Epub 2023 Mar 12. Expert Opin Drug Deliv. 2023. PMID: 36896650 Free PMC article. Review.
-
Aerosolised micro and nanoparticle: formulation and delivery method for lung imaging.Clin Transl Imaging. 2023;11(1):33-50. doi: 10.1007/s40336-022-00527-3. Epub 2022 Sep 29. Clin Transl Imaging. 2023. PMID: 36196096 Free PMC article. Review.
-
Development of Spray-Dried Micelles, Liposomes, and Solid Lipid Nanoparticles for Enhanced Stability.Pharmaceutics. 2025 Jan 17;17(1):122. doi: 10.3390/pharmaceutics17010122. Pharmaceutics. 2025. PMID: 39861769 Free PMC article.
-
Approaches and applications in transdermal and transpulmonary gene drug delivery.Front Bioeng Biotechnol. 2025 Jan 15;12:1519557. doi: 10.3389/fbioe.2024.1519557. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39881959 Free PMC article. Review.
-
Targeted Molecular Therapeutics for Pulmonary Diseases: Addressing the Need for Precise Drug Delivery.Handb Exp Pharmacol. 2024;284:313-328. doi: 10.1007/164_2023_703. Handb Exp Pharmacol. 2024. PMID: 38177399
References
-
- Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med. 2018;20(5):e3015. - PubMed
-
- Mccarthy HO, McCaffrey J, Mccrudden CM, Zholobenko A, Ali AA, McBride JW, et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J Control Release. Elsevier B.V. 2014;189:141–149. doi: 10.1016/j.jconrel.2014.06.048. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

