Preclinical efficacy and safety of novel SNAT against SARS-CoV-2 using a hamster model
- PMID: 35441321
- PMCID: PMC9017740
- DOI: 10.1007/s13346-022-01166-x
Preclinical efficacy and safety of novel SNAT against SARS-CoV-2 using a hamster model
Abstract
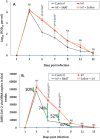
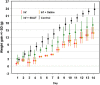
To address the unprecedented global public health crisis due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), we designed and developed a novel antiviral nano-drug, called SNAT (Smart Nano-Enabled Antiviral Therapeutic), comprised of taxoid (Tx)-decorated amino (NH2)-functionalized near-atomic size positively charged silver nanoparticles (Tx-[NH2-AgNPs]) that are stable for over 3 years. Using a hamster model, we tested the preclinical efficacy of inhaled SNAT on the body weight, virus titer, and histopathology of lungs in SARS-CoV-2-infected hamsters, including biocompatibility in human lung epithelium and dermal fibroblasts using lactase dehydrogenase (LDH) and malondialdehyde (MDA) assays. Our results showed SNAT could effectively reverse the body weight loss, reduce the virus load in oral swabs, and improve lung health in hamsters. Furthermore, LDH assay showed SNAT is noncytotoxic, and MDA assay demonstrated SNAT to be an antioxidant, potentially quenching lipid peroxidation, in both the human cells. Overall, these promising pilot preclinical findings suggest SNAT as a novel, safer antiviral drug lead against SARS-CoV-2 infection and may find applications as a platform technology against other respiratory viruses of epidemic and pandemic potential.
Keywords: Antiviral drug candidate; COVID-19; Inhaler; Nanotechnology; SARS-CoV-2; SNAT.
© 2022. Controlled Release Society.
Conflict of interest statement
All the patents’ rights (US Patent No. 63/042,070, and US Patent No. PCT/US2021/014343) are owned by East Carolina University and L.R.P. is the sole inventor.
Figures




References
-
- World Health Organization. Severe acute respiratory syndrome (SARS). WHO 2022. Available at: https://www.who.int/health-topics/severe-acute-respiratory-syndrome#tab=.... Accessed 20 Dec 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

