Chimeric antigen receptor clustering via cysteines enhances T-cell efficacy against tumor
- PMID: 35441325
- PMCID: PMC10992513
- DOI: 10.1007/s00262-022-03195-4
Chimeric antigen receptor clustering via cysteines enhances T-cell efficacy against tumor
Abstract
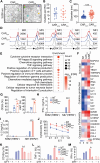
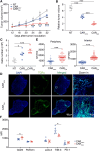
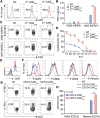
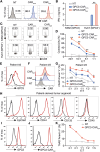
Chimeric antigen receptor (CAR) T-cell therapy achieves great success for hematological malignancies. However, clinical trials have revealed some limitations in both improving the efficacy and reducing the relapse, which calls for innovative strategies to engineer more powerful CAR-T cells. Promoting the formation of CAR clusters provides an alternative approach and potentially improves current CAR T-cell therapy against cancers. Here, we generated CARCys-T cells using a 4-1BB-derived hinge region including 11 cysteines residues. The cysteines in the hinge were found to facilitate CARCys clustering upon antigen stimulation and promote the antitumor activity of CAR-T cells. Compared with most conventionally used CAR-T cells with CD8α-derived hinge (CARconv-T cells), CARCys-T cells exhibited larger diameter of CAR clusters and enhanced antigen-specific tumor lysis both in vitro and in vivo. In addition, the CARCys-mediated enhancement could be applied to HER2, CD19 as well as GPC3-targeted CAR-T cells. More importantly, CARCys-T cells showed potent antitumor efficacy in clinically relevant patient-derived primary tumor cells and organoids. Thus, the novel hinge containing 11 cysteines provides a promising strategy to facilitate CAR clustering and maximize anti-tumor activity of CAR-T cells, which emphasizes the importance of CAR clustering to improve CAR T-cell therapy in the clinic.
Keywords: CAR clustering; CAR-T cell; Cancer immunotherapy; Cysteines; Hinge domain.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

