TOR inhibition primes immunity and pathogen resistance in tomato in a salicylic acid-dependent manner
- PMID: 35441436
- PMCID: PMC9190978
- DOI: 10.1111/mpp.13207
TOR inhibition primes immunity and pathogen resistance in tomato in a salicylic acid-dependent manner
Abstract
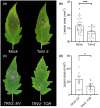
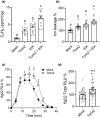
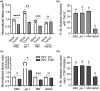
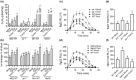
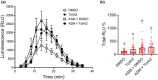
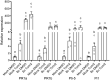
All organisms need to sense and process information about the availability of nutrients, energy status, and environmental cues to determine the best time for growth and development. The conserved target of rapamycin (TOR) protein kinase has a central role in sensing and perceiving nutritional information. TOR connects environmental information about nutrient availability to developmental and metabolic processes to maintain cellular homeostasis. Under favourable energy conditions, TOR is activated and promotes anabolic processes such as cell division, while suppressing catabolic processes. Conversely, when nutrients are limited or environmental stresses are present, TOR is inactivated, and catabolic processes are promoted. Given the central role of TOR in regulating metabolism, several previous works have examined whether TOR is wired to plant defence. To date, the mechanisms by which TOR influences plant defence are not entirely clear. Here, we addressed this question by testing the effect of inhibiting TOR on immunity and pathogen resistance in tomato. Examining which hormonal defence pathways are influenced by TOR, we show that tomato immune responses and disease resistance to several pathogens increase on TOR inhibition, and that TOR inhibition-mediated resistance probably requires a functional salicylic acid, but not jasmonic acid, pathway. Our results support the notion that TOR is a master regulator of the development-defence switch in plants.
Keywords: Botrytis; Xanthomonas; TOR; immunity; pathogenesis; tobacco mosaic virus; tomato.
© 2022 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Brading, P.A. , Hammond‐Kosack, K.E. , Parr, A. & Jones, J.D.G. (2000) Salicylic acid is not required for Cf‐2‐ and Cf‐9‐dependent resistance of tomato to Cladosporium fulvum . The Plant Journal, 23, 305–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

