Therapeutic stem cell-derived alveolar-like macrophages display bactericidal effects and resolve Pseudomonas aeruginosa-induced lung injury
- PMID: 35441437
- PMCID: PMC9097833
- DOI: 10.1111/jcmm.17324
Therapeutic stem cell-derived alveolar-like macrophages display bactericidal effects and resolve Pseudomonas aeruginosa-induced lung injury
Abstract
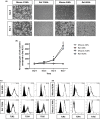
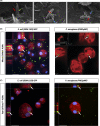
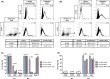
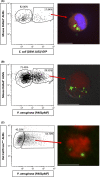
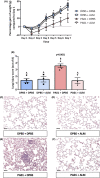
Bacterial lung infections lead to greater than 4 million deaths per year with antibiotic treatments driving an increase in antibiotic resistance and a need to establish new therapeutic approaches. Recently, we have generated mouse and rat stem cell-derived alveolar-like macrophages (ALMs), which like primary alveolar macrophages (1'AMs), phagocytose bacteria and promote airway repair. Our aim was to further characterize ALMs and determine their bactericidal capabilities. The characterization of ALMs showed that they share known 1'AM cell surface markers, but unlike 1'AMs are highly proliferative in vitro. ALMs effectively phagocytose and kill laboratory strains of P. aeruginosa (P.A.), E. coli (E.C.) and S. aureus, and clinical strains of P.A. In vivo, ALMs remain viable, adapt additional features of native 1'AMs, but proliferation is reduced. Mouse ALMs phagocytose P.A. and E.C. and rat ALMs phagocytose and kill P.A. within the lung 24 h post-instillation. In a pre-clinical model of P.A.-induced lung injury, rat ALM administration mitigated weight loss and resolved lung injury observed seven days post-instillation. Collectively, ALMs attenuate pulmonary bacterial infections and promote airway repair. ALMs could be utilized as an alternative or adjuvant therapy where current treatments are ineffective against antibiotic-resistant bacteria or to enhance routine antibiotic delivery.
Keywords: alveolar macrophage; antibiotic resistance; bacterial lung injury; bactericidal effects; pluripotent stem cell.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures


References
-
- Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11:404‐406. - PubMed
-
- Ciofu O, Hansen CR, Høiby N. Respiratory bacterial infections in cystic fibrosis. Curr Opin Pulm Med. 2013;19:251‐258. - PubMed
-
- Raju R, Peters BS, Breen RAM. Lung infections in the HIV‐infected adult. Curr Opin Pulm Med. 2012;18:253‐258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

