Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension
- PMID: 35441470
- PMCID: PMC9542252
- DOI: 10.1111/dom.14725
Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension
Abstract
Aim: To explore changes in body weight and cardiometabolic risk factors after treatment withdrawal in the STEP 1 trial extension.
Materials and methods: STEP 1 (NCT03548935) randomized 1961 adults with a body mass index ≥ 30 kg/m2 (or ≥ 27 kg/m2 with ≥ 1 weight-related co-morbidity) without diabetes to 68 weeks of once-weekly subcutaneous semaglutide 2.4 mg (including 16 weeks of dose escalation) or placebo, as an adjunct to lifestyle intervention. At week 68, treatments (including lifestyle intervention) were discontinued. An off-treatment extension assessed for a further year a representative subset of participants who had completed 68 weeks of treatment. This subset comprised all eligible participants from any site in Canada, Germany and the UK, and sites in the United States and Japan with the highest main phase recruitment. All analyses in the extension were exploratory.
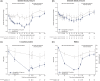
Results: Extension analyses included 327 participants. From week 0 to week 68, mean weight loss was 17.3% (SD: 9.3%) with semaglutide and 2.0% (SD: 6.1%) with placebo. Following treatment withdrawal, semaglutide and placebo participants regained 11.6 (SD: 7.7) and 1.9 (SD: 4.8) percentage points of lost weight, respectively, by week 120, resulting in net losses of 5.6% (SD: 8.9%) and 0.1% (SD: 5.8%), respectively, from week 0 to week 120. Cardiometabolic improvements seen from week 0 to week 68 with semaglutide reverted towards baseline at week 120 for most variables.
Conclusions: One year after withdrawal of once-weekly subcutaneous semaglutide 2.4 mg and lifestyle intervention, participants regained two-thirds of their prior weight loss, with similar changes in cardiometabolic variables. Findings confirm the chronicity of obesity and suggest ongoing treatment is required to maintain improvements in weight and health.
Keywords: GLP-1 analogue; antiobesity drug; clinical trial; obesity therapy; phase III study; weight control.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
JPHW reports receiving advisory board fees, paid to his institution, from Astellas Pharma, grant support and fees for membership on a data and safety monitoring board, both paid to the University of Liverpool, lecture fees and travel support from AstraZeneca, advisory board fees, paid to his institution, and lecture fees from Boehringer Ingelheim, Napp and Sanofi Pasteur, advisory board fees, paid to his institution, from Eli Lilly, Janssen Global Services, Rhythm and Wilmington Healthcare, lecture fees from Mundipharma, grant support, advisory board fees and fees for serving as an investigator, all paid to the University of Liverpool, and lecture fees from Novo Nordisk and advisory board fees from Takeda Medical Research Foundation. RLB reports research grant support from Novo Nordisk and consultancy with Boehringer Ingelheim, Eli Lilly, Gila Therapeutics Inc, GSK, Novo Nordisk, and Pfizer. MD reports receiving research funding from AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk and Sanofi‐Aventis, and has acted as a consultant, advisory board member and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi‐Aventis, as an advisory board member and speaker for AstraZeneca, as an advisory board member for Gilead Sciences Ltd, Janssen and Lexicon, and as a speaker for Napp Pharmaceuticals and Takeda Pharmaceuticals International Inc. She is co‐funded by the NIHR Leicester Biomedical Research Centre. LFVG reports receiving lecture fees from AstraZeneca and Boehringer Ingelheim and advisory board fees and lecture fees from Merck and Novo Nordisk. KKa reports being employed by and owning stock in Novo Nordisk. KKo and TKO report being employed by Novo Nordisk. IL reports receiving advisory board fees and/or consulting fees from AstraZeneca, Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, Eli Lilly, Intarcia, Intercept Pharmaceuticals, Janssen Global Services, MannKind, Novo Nordisk, Sanofi, Target Pharma, Valeritas and Zealand Pharma, and grant support, paid to UT Southwestern, from Merck, Mylan Pharmaceuticals, Novo Nordisk, Pfizer and Sanofi. BMM reports receiving educational fees from AstraZeneca, Merck and Orexigen Therapeutics, lecture fees from Janssen Biotech, advisory board fees from Johnson & Johnson Health Care Systems, grant support, paid to Guy's and St. Thomas' Hospital, consulting fees and educational fees from Novo Nordisk and owning stock in Reset Health Clinics. JR reports receiving grant support, advisory board fees and travel support from Applied Therapeutics, Intarcia and Oramed, grant support and consulting fees from AstraZeneca, grant support, advisory board fees, lecture fees and travel support from Boehringer Ingelheim, Novo Nordisk and Sanofi US Services, grant support and advisory board fees from Eli Lilly, grant support from Genentech, GlaxoSmithKline, Janssen Biotech, Lexicon Pharmaceuticals, Novartis, Pfizer and REMD Biotherapeutics and advisory board fees from Zealand Pharma. TAW reports receiving grant support from Novo Nordisk and Epitomee Medical, paid to the University of Pennsylvania, and personal advisory board fees from Novo Nordisk and WW International. SW reports receiving lecture fees from AstraZeneca and Bausch and Lomb and grant support, lecture fees and advisory board fees from Novo Nordisk. KY reports receiving lecture fees from Amgen, Janssen Pharmaceuticals, Kyowa Hakko Kirin, Novartis Pharma and Sanofi, grant support and lecture fees from Astellas Pharma, Daiichi Sankyo, Eli Lilly Japan, Merck Sharp and Dohme, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novo Nordisk, Ono Pharmaceutical, Pfizer, Sumitomo Dainippon Pharma, Taisho Toyama Pharmaceutical and Takeda Pharmaceutical, advisory board fees and lecture fees from AstraZeneca, grant support, lecture fees and advisory board fees from Kowa Company and Novo Nordisk and lecture fees and advisory board fees from Sanofi. RFK reports receiving advisory board fees from Novo Nordisk and WW International.
Figures


References
-
- Bessesen DH, Van Gaal LF. Progress and challenges in anti‐obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6:237‐248. - PubMed
-
- Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1‐203. - PubMed
-
- World Health Organization . Obesity and overweight. 2020. https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight. Accessed January 11, 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

