CD38 Drives Progress of Osteoarthritis by Affecting Cartilage Homeostasis
- PMID: 35441488
- PMCID: PMC9087467
- DOI: 10.1111/os.13258
CD38 Drives Progress of Osteoarthritis by Affecting Cartilage Homeostasis
Abstract
Objective: To observe expression of CD38, a key modulator of nicotinamide dinucleotide (NAD+) metabolism in mice with knee osteoarthritis, and protective effect of CD38 inhibition during the osteoarthritis (OA) development.
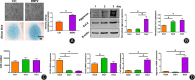
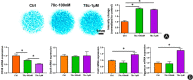
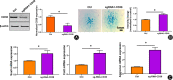
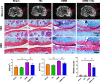
Method: The destabilization of the medial meniscus (DMM) model was performed in mice to mimic the process of OA. Immunofluorescence of CD38 was performed to evaluate its response during the OA process. Limb bud-derived mesenchymal cells were isolated for micromass culture. 100 nM or 1 μM CD38 inhibitor (78c) treatment for 14 days and CD38 sgRNA infection were then used to explore the effects of chondrogenic differentiation via Alcian blue staining. The expressions of chondrogenic markers were detected using RT-PCR and Western blot. To explore the protective effect of CD38 inhibitor on cartilage degradation during OA in vivo, a CD38 inhibitor was injected into the knee joint after DMM operations. Micro-CT analysis and Safranin O-fast green staining were used to evaluate subchondral bone micro-architecture changes and cartilage degeneration.
Results: Compared to the control group, the CD38 expression in superficial cartilage was obviously increased in DMM group (P < 0.05). During the normal chondrogenic differentiation, the extracellular matrix formed and expression of Sox9, Col2, aggrecan increased apparently while CD38 expression decreased, which could be reversed with ablation of CD38 in limb bud-derived mesenchymal cells. Consistent with findings in vitro, CD38 blockage via CD38 inhibitor injection protected against osteosclerosis in medial subchondral bone and cartilage degeneration in DMM-induced experimental mice. Compared to the Sham group, DMM mice showed significantly increased values of BV and BV/TV in subchondral bone (P < 0.05) and Mankin score, which could be rescued by 78c treatment (P < 0.05). Also the CD38 inhibitor contributed to homeostasis of anabolism and catabolism by upregulating Sox9, Col2, aggrecan and downregulating Runx2, Col10 and Mmp13.
Conclusion: This study primarily implicates CD38 as an important regulator of chondrogenic differentiation. Inhibition of CD38 demonstrated protection against cartilage degeneration, which suggests that CD38 could be a potential therapeutic target for OA.
Keywords: CD38; Cartilage; Chondrocyte; NAD; Osteoarthritis.
© 2022 The Authors. Orthopaedic Surgery published by Tianjin Hospital and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors have declared that there is no conflict of interests in this work.
Figures


Similar articles
-
Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes.J Ethnopharmacol. 2020 Jan 30;247:112261. doi: 10.1016/j.jep.2019.112261. Epub 2019 Sep 29. J Ethnopharmacol. 2020. PMID: 31577939
-
Teriparatide ameliorates articular cartilage degradation and aberrant subchondral bone remodeling in DMM mice.J Orthop Translat. 2022 Dec 7;38:241-255. doi: 10.1016/j.jot.2022.10.015. eCollection 2023 Jan. J Orthop Translat. 2022. PMID: 36514714 Free PMC article.
-
Proximal fibular osteotomy alleviates medial compartment knee osteoarthritis in a mouse model.Int Orthop. 2020 Jun;44(6):1107-1113. doi: 10.1007/s00264-020-04497-x. Epub 2020 Feb 10. Int Orthop. 2020. PMID: 32040598
-
The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases.Front Immunol. 2019 May 31;10:1187. doi: 10.3389/fimmu.2019.01187. eCollection 2019. Front Immunol. 2019. PMID: 31214171 Free PMC article. Review.
-
The Homeostasis of Cartilage Matrix Remodeling and the Regulation of Volume-Sensitive Ion Channel.Aging Dis. 2022 Jun 1;13(3):787-800. doi: 10.14336/AD.2021.1122. eCollection 2022 Jun. Aging Dis. 2022. PMID: 35656105 Free PMC article. Review.
Cited by
-
Immunogenicity of chondrocyte sheets: a review.Front Immunol. 2025 Mar 7;16:1529384. doi: 10.3389/fimmu.2025.1529384. eCollection 2025. Front Immunol. 2025. PMID: 40124370 Free PMC article. Review.
-
Sox, Fox, and Lmx1b binding sites differentially regulate a Gdf5-Associated regulatory region during elbow development.Front Cell Dev Biol. 2023 Jul 10;11:1215406. doi: 10.3389/fcell.2023.1215406. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37492222 Free PMC article.
-
Bionic bearing-inspired lubricating microspheres with Immunomodulatory effects for osteoarthritis therapy.J Nanobiotechnology. 2025 Jun 20;23(1):457. doi: 10.1186/s12951-025-03544-2. J Nanobiotechnology. 2025. PMID: 40542398 Free PMC article.
-
Targeting rheumatoid arthritis: a molecular perspective on biologic therapies and clinical progress.J Biol Eng. 2025 Jul 24;19(1):67. doi: 10.1186/s13036-025-00534-8. J Biol Eng. 2025. PMID: 40707975 Free PMC article. Review.
References
-
- Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009;35:579–90. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

