Neocortical pyramidal neurons with axons emerging from dendrites are frequent in non-primates, but rare in monkey and human
- PMID: 35441590
- PMCID: PMC9159751
- DOI: 10.7554/eLife.76101
Neocortical pyramidal neurons with axons emerging from dendrites are frequent in non-primates, but rare in monkey and human
Abstract
The canonical view of neuronal function is that inputs are received by dendrites and somata, become integrated in the somatodendritic compartment and upon reaching a sufficient threshold, generate axonal output with axons emerging from the cell body. The latter is not necessarily the case. Instead, axons may originate from dendrites. The terms 'axon carrying dendrite' (AcD) and 'AcD neurons' have been coined to describe this feature. In rodent hippocampus, AcD cells are shown to be functionally 'privileged', since inputs here can circumvent somatic integration and lead to immediate action potential initiation in the axon. Here, we report on the diversity of axon origins in neocortical pyramidal cells of rodent, ungulate, carnivore, and primate. Detection methods were Thy-1-EGFP labeling in mouse, retrograde biocytin tracing in rat, cat, ferret, and macaque, SMI-32/βIV-spectrin immunofluorescence in pig, cat, and macaque, and Golgi staining in macaque and human. We found that in non-primate mammals, 10-21% of pyramidal cells of layers II-VI had an AcD. In marked contrast, in macaque and human, this proportion was lower and was particularly low for supragranular neurons. A comparison of six cortical areas (being sensory, association, and limbic in nature) in three macaques yielded percentages of AcD cells which varied by a factor of 2 between the areas and between the individuals. Unexpectedly, pyramidal cells in the white matter of postnatal cat and aged human cortex exhibit AcDs to much higher percentages. In addition, interneurons assessed in developing cat and adult human cortex had AcDs at type-specific proportions and for some types at much higher percentages than pyramidal cells. Our findings expand the current knowledge regarding the distribution and proportion of AcD cells in neocortex of non-primate taxa, which strikingly differ from primates where these cells are mainly found in deeper layers and white matter.
Keywords: axon initial segment; evolution; evolutionary biology; human; inhibitory interneurons; interstitial cells; mouse; neurofilament; neuroscience; rat; rhesus macaque; subplate.
© 2022, Wahle et al.
Conflict of interest statement
PW, ES, IG, NL, SW, JL, ME, CD, GM No competing interests declared
Figures



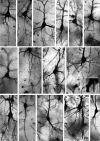





Comment in
- doi: 10.7554/eLife.79839
References
-
- Benavides-Piccione R, Regalado-Reyes M, Fernaud-Espinosa I, Kastanauskaite A, Tapia-González S, León-Espinosa G, Rojo C, Insausti R, Segev I, DeFelipe J. Differential Structure of Hippocampal CA1 Pyramidal Neurons in the Human and Mouse. Cerebral Cortex (New York, N.Y. 2020;30:730–752. doi: 10.1093/cercor/bhz122. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

