Discrimination of RNA fiber structures using solid-state nanopores
- PMID: 35441627
- PMCID: PMC9520586
- DOI: 10.1039/d1nr08002d
Discrimination of RNA fiber structures using solid-state nanopores
Abstract
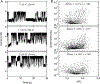
RNA fibers are a class of biomaterials that can be assembled using HIV-like kissing loop interactions. Because of the programmability of molecular design and low immunorecognition, these structures present an interesting opportunity to solve problems in nanobiotechnology and synthetic biology. However, the experimental tools to fully characterize and discriminate among different fiber structures in solution are limited. Herein, we utilize solid-state nanopore experiments and Brownian dynamics simulations to characterize and distinguish several RNA fiber structures that differ in their degrees of branching. We found that, regardless of the electrolyte type and concentration, fiber structures that have more branches produce longer and deeper ionic current blockades in comparison to the unbranched fibers. Experiments carried out at temperatures ranging from 20-60 °C revealed almost identical distributions of current blockade amplitudes, suggesting that the kissing loop interactions in fibers are resistant to heating within this range.
Figures

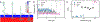




References
-
- Saito RF; Rangel MC; Halman JR; Chandler M; de Sousa Andrade LN; Odete-Bustos S; Furuya TK; Carrasco AGM; Chaves-Filho AB; Yoshinaga MY; Miyamoto S; Afonin KA; Chammas R, Simultaneous silencing of Lysophosphatidylcholine Acyltransferases 1–4 by nucleic acid nanoparticles (NANPs) improves radiation response of melanoma cells. Nanomedicine: Nanotechnology, Biology and Medicine 2021, 102418. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

