Solvent-free amide bond formation using a variety of methoxysilanes as coupling agent
- PMID: 35441639
- PMCID: PMC9092949
- DOI: 10.1039/d2ob00589a
Solvent-free amide bond formation using a variety of methoxysilanes as coupling agent
Abstract
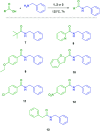
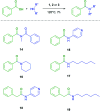
A solvent-free procedure for the formation of amides without exclusion of air and moisture is described. Using tetramethoxysilane 1, hexamethoxydisilane 2 and dodecamethoxy-neopentasilane 3 as coupling agent carboxylic acids and amines are reacted to form amides in good to excellent yields. The formation of these amides was confirmed by NMR spectroscopy and mass spectrometry. Remarkably, neopentasilane 3 exceeds the performance of the currently used monosilanes as coupling agent in terms of group tolerance and yield.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

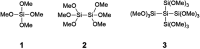
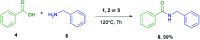

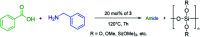
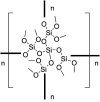
Similar articles
-
One-pot mechanosynthesis of aromatic amides and dipeptides from carboxylic acids and amines.Chem Commun (Camb). 2012 Dec 25;48(99):12100-2. doi: 10.1039/c2cc36613d. Epub 2012 Nov 8. Chem Commun (Camb). 2012. PMID: 23135220
-
B(OCH2CF3)3-mediated direct amidation of pharmaceutically relevant building blocks in cyclopentyl methyl ether.Org Biomol Chem. 2015 Nov 28;13(44):10888-94. doi: 10.1039/c5ob01801c. Epub 2015 Sep 14. Org Biomol Chem. 2015. PMID: 26366853
-
Triphenylphosphine-catalysed amide bond formation between carboxylic acids and amines.Chem Commun (Camb). 2014 Jun 1;50(43):5763-6. doi: 10.1039/c4cc01861c. Epub 2014 Apr 22. Chem Commun (Camb). 2014. PMID: 24752820
-
Harnessing and engineering amide bond forming ligases for the synthesis of amides.Curr Opin Chem Biol. 2020 Apr;55:77-85. doi: 10.1016/j.cbpa.2019.12.004. Epub 2020 Feb 12. Curr Opin Chem Biol. 2020. PMID: 32058241 Review.
-
Recent Advances in the Metal-Catalyzed Activation of Amide Bonds.Chem Asian J. 2019 Jan 4;14(1):76-93. doi: 10.1002/asia.201801317. Epub 2018 Dec 6. Chem Asian J. 2019. PMID: 30426696 Review.
Cited by
-
On the Use of Triarylsilanols as Catalysts for Direct Amidation of Carboxylic Acids.J Org Chem. 2023 Jul 21;88(14):9853-9869. doi: 10.1021/acs.joc.3c00585. Epub 2023 Jul 11. J Org Chem. 2023. PMID: 37432502 Free PMC article.
References
-
- Roughley S. D. Jordan A. M. J. Med. Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. - DOI - PubMed
- Bryan M. C. Dunn P. J. Entwistle D. Gallou F. Koenig S. G. Hayler J. D. Hickey M. R. Hughes S. Kopach M. E. Moine G. Richardson P. Roschangar F. Steven A. Weiberth F. J. Green Chem. 2018;20:5082–5103. doi: 10.1039/C8GC01276H. - DOI
-
- Modern Crop Protection Compounds, ed. P. Jeschke, M. Witschel, W. Krämer and U. Schirmer, Wiley-VCH, Weinheim, 3rd edn, 2018
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

