Risk factors for severe reactions in food allergy: Rapid evidence review with meta-analysis
- PMID: 35441718
- PMCID: PMC9544052
- DOI: 10.1111/all.15318
Risk factors for severe reactions in food allergy: Rapid evidence review with meta-analysis
Abstract
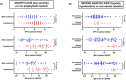
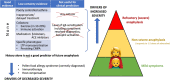
This rapid review summarizes the most up to date evidence about the risk factors for severe food-induced allergic reactions. We searched three bibliographic databases for studies published between January 2010 and August 2021. We included 88 studies and synthesized the evidence narratively, undertaking meta-analysis where appropriate. Significant uncertainties remain with respect to the prediction of severe reactions, both anaphylaxis and/or severe anaphylaxis refractory to treatment. Prior anaphylaxis, an asthma diagnosis, IgE sensitization or basophil activation tests are not good predictors. Some molecular allergology markers may be helpful. Hospital presentations for anaphylaxis are highest in young children, yet this age group appears at lower risk of severe outcomes. Risk of severe outcomes is greatest in adolescence and young adulthood, but the contribution of risk taking behaviour in contributing to severe outcomes is unclear. Evidence for an impact of cofactors on severity is lacking, although food-dependent exercise-induced anaphylaxis may be an exception. Some medications such as beta-blockers or ACE inhibitors may increase severity, but appear less important than age as a factor in life-threatening reactions. The relationship between dose of exposure and severity is unclear. Delays in symptom recognition and anaphylaxis treatment have been associated with more severe outcomes. An absence of prior anaphylaxis does not exclude its future risk.
Keywords: anaphylaxis; biomarkers; food allergy; risk assessment; severity.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Dr Turner reports personal fees from Aimmune Therapeutics, DBV Technologies, Allergenis, UK Food Standards Agency and ILSI Europe; grants from National Institute for Health Research (NIHR)/Imperial Biomedical Research Centre, UK Medical Research Council, UK Food Standards Agency, End Allergies Together, Jon Moulton Charity Trust, outside the submitted work. Prof Halken reports speaker/chair fees from Nestlé Purina and ABIGO Pharma A/S. Dr Muraro reports speaker fees from Aimmune Therapeutics, DBV, and Nestlé Purina; and is an advisory board member/fees from Regeneron IDMC. Dr Deschildre reports personal fees from ALK, Stallergènes‐Greer, GSK, Novartis, Sanofi, Regeneron, Bohringer Ingelheim, Aimmune therapeutics, DBV technologies, Nestlé Health Science, Nutricia, outside the submitted work. Dr Ballmer‐Weber reports personal fees from ALK, Allergopharma, ThermoFisher, Menarini, Sanofi, Novartis. Prof. van Ree reports personal fees from HAL Allergy, ALK, Citeq, Angany, ThermoFisher, Reacta Healthcare, and Mission MightyMe; grants from European Commission, Dutch Science Foundation, and Health Holland, outside the submitted work. Prof. Worm declares the receipt of honoraria or consultation fees by the following companies: ALK‐Abelló Arzneimittel GmbH, Mylan Germany GmbH, Leo Pharma GmbH, Sanofi‐Aventis Deutschland GmbH, Regeneron Pharmaceuticals, DBV Technologies S.A, Stallergenes GmbH, HAL Allergie GmbH, Allergopharma GmbH & Co.KG, Bencard Allergie GmbH, Aimmune Therapeutics UK Limited, Actelion Pharmaceuticals Deutschland GmbH, Novartis AG, Biotest AG., AbbVie Deutschland GmbH & Co. KG and Lilly Deutschland GmbH, outside the submitted work. Prof Zuberbier reports personal fees from Bayer, FAES, Novartis, Henkel, AstraZeneca, AbbVie, ALK, Almirall, Astellas, Bencard, Berlin Chemie, HAL, Leti, Meda, Menarini, Merck, MSD, Pfizer, Sanofi, Stallergenes, Takeda, Teva, UCB, Henkel, Kryolan, L’Oréal, outside the submitted work. Prof Roberts reports grants from UK Food Standards Agency, DBV and the European Union. The other authors have no relevant conflicts of interest to declare.
Figures


References
-
- Vyas D, Ierodiakonou D, Harrison DA, Russell T, Turner PJ, Boyle RJ. Increase in intensive care unit admissions for anaphylaxis in the United Kingdom 2008–2012. J Allergy Clin Immunol. 2016;137(2):AB57.
-
- Turner PJ, Baumert JL, Beyer K, et al. Can we identify patients at risk of life‐threatening allergic reactions to food? Allergy. 2016;71(9):1241‐1255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

