Evaluation of Individual Items of the Patient Health Questionnaire (PHQ-9) and Montgomery-Asberg Depression Rating Scale (MADRS) in Adults with Treatment-Resistant Depression Treated with Esketamine Nasal Spray Combined with a New Oral Antidepressant
- PMID: 35441931
- PMCID: PMC9166828
- DOI: 10.1007/s40263-022-00916-2
Evaluation of Individual Items of the Patient Health Questionnaire (PHQ-9) and Montgomery-Asberg Depression Rating Scale (MADRS) in Adults with Treatment-Resistant Depression Treated with Esketamine Nasal Spray Combined with a New Oral Antidepressant
Abstract
Objective: The objective of this study was to determine which symptoms measured by the Patient Health Questionnaire (PHQ-9) and Montgomery-Asberg Depression Rating Scale (MADRS) improve in those treated with esketamine nasal spray in combination with oral antidepressant (AD) compared with those treated with placebo plus AD for adult patients with treatment-resistant depression (TRD). These results complement the interpretation of PHQ-9 and MADRS total scores.
Methods: The TRANSFORM 2 study evaluated the efficacy and safety of esketamine nasal spray in combination with AD. This post-hoc analysis used PHQ-9 and MADRS data to evaluate symptom changes. The total scores change and proportions of individual item change scores on the PHQ-9 and MADRS were evaluated at days 15 and 28; analysis of variance was used to test differences on total scores. Generalized estimation equations of logistic regression models were used to estimate the likelihood of improvement on instrument items.
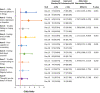
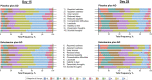
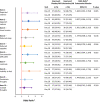
Results: The mean total score reduction of the PHQ-9, indicating improvement, was greater in the esketamine plus AD arm compared with placebo plus AD at day 15 (- 1.8; p = 0.045) and day 28 (- 2.8; p = 0.006). Proportions of those who improved (≥ 1 point on a 4-point scale and ≥ 2 points on a 7-point scale for the PHQ-9 and MADRS, respectively) was greater in the esketamine plus AD group compared with the placebo plus AD group across all items. The odds of improving for those in the esketamine plus AD group compared with the placebo plus AD group were over two times greater on the PHQ-9 items: "Little interest/pleasure in things" (OR 2.252, 95% CI 1.165-4.355); "Feeling down, depressed, or hopeless" (OR 2.767, 95% CI 1.400-5.470); and "Feeling tired or having little energy" (OR 2.171, 95% CI 1.153-4.087). The mean reduction in total scores on the MADRS, indicating improvement, was numerically greater at day 15 (- 2.0; p = 0.189) and statistically significantly greater at day 28 (- 4.4; p = 0.017) in the esketamine plus AD arm compared with placebo plus AD. The odds of improving for those in the esketamine plus AD group compared with the placebo plus AD group were over two times greater on the MADRS items measuring "Apparent sadness" (OR 2.007, 95% CI 1.096-3.674); and "Inability to feel" (OR 2.099, 95% CI 1.180-3.735).
Conclusion: Improvement in mean total scores in those treated with esketamine plus AD compared with placebo plus AD are important results to confirm efficacy. The odds of improving in those treated with esketamine plus AD was at least two times greater than with placebo plus AD on three patient- and two clinician-reported individual symptoms of TRD. These findings provide patient-relevant quantification of the esketamine plus AD treatment benefit, adding understanding as to which symptoms are most improved with treatment.
Trial registration: ClinicalTrials.gov identifier: NCT02418585; first posted 16 April 2015.
© 2022. The Author(s).
Conflict of interest statement
CJ, VP, WD, KC, and JS are employees of Janssen Pharmaceutical Companies of Johnson & Johnson. LF and SH declare no competing interests.
Figures

References
-
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

