Engineering Isopropanol Dehydrogenase for Efficient Regeneration of Nicotinamide Cofactors
- PMID: 35442081
- PMCID: PMC9088361
- DOI: 10.1128/aem.00341-22
Engineering Isopropanol Dehydrogenase for Efficient Regeneration of Nicotinamide Cofactors
Abstract
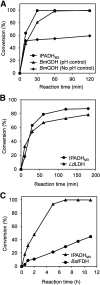
Isopropanol dehydrogenase (IPADH) is one of the most attractive options for nicotinamide cofactor regeneration due to its low cost and simple downstream processing. However, poor thermostability and strict cofactor dependency hinder its practical application for bioconversions. In this study, we simultaneously improved the thermostability (433-fold) and catalytic activity (3.3-fold) of IPADH from Brucella suis via a flexible segment engineering strategy. Meanwhile, the cofactor preference of IPADH was successfully switched from NAD(H) to NADP(H) by 1.23 × 106-fold. When these variants were employed in three typical bioredox reactions to drive the synthesis of important chiral pharmaceutical building blocks, they outperformed the commonly used cofactor regeneration systems (glucose dehydrogenase [GDH], formate dehydrogenase [FDH], and lactate dehydrogenase [LDH]) with respect to efficiency of cofactor regeneration. Overall, our study provides two promising IPADH variants with complementary cofactor specificities that have great potential for wide applications. IMPORTANCE Oxidoreductases represent one group of the most important biocatalysts for synthesis of various chiral synthons. However, their practical application was hindered by the expensive nicotinamide cofactors used. Isopropanol dehydrogenase (IPADH) is one of the most attractive biocatalysts for nicotinamide cofactor regeneration. However, poor thermostability and strict cofactor dependency hinder its practical application. In this work, the thermostability and catalytic activity of an IPADH were simultaneously improved via a flexible segment engineering strategy. Meanwhile, the cofactor preference of IPADH was successfully switched from NAD(H) to NADP(H). The resultant variants show great potential for regeneration of nicotinamide cofactors, and the engineering strategy might serve as a useful approach for future engineering of other oxidoreductases.
Keywords: cofactor regeneration; cofactor specificity reversal; isopropanol dehydrogenase; protein engineering; thermostability evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Regeneration of nicotinamide coenzymes: principles and applications for the synthesis of chiral compounds.Adv Biochem Eng Biotechnol. 2010;120:195-242. doi: 10.1007/10_2009_55. Adv Biochem Eng Biotechnol. 2010. PMID: 20182929
-
Engineering a Formate Dehydrogenase for NADPH Regeneration.Chembiochem. 2023 Oct 17;24(20):e202300390. doi: 10.1002/cbic.202300390. Epub 2023 Aug 28. Chembiochem. 2023. PMID: 37455264
-
Structure-Guided Design of Formate Dehydrogenase for Regeneration of a Non-Natural Redox Cofactor.Chemistry. 2020 Dec 15;26(70):16611-16615. doi: 10.1002/chem.202003102. Epub 2020 Nov 16. Chemistry. 2020. PMID: 32815230
-
[Formate dehydrogenase and its application in biomanufacturing of chiral chemicals].Sheng Wu Gong Cheng Xue Bao. 2022 Feb 25;38(2):632-649. doi: 10.13345/j.cjb.210278. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 35234387 Review. Chinese.
-
Redox Biocatalysis: Quantitative Comparisons of Nicotinamide Cofactor Regeneration Methods.ChemSusChem. 2022 Nov 22;15(22):e202200888. doi: 10.1002/cssc.202200888. Epub 2022 Oct 26. ChemSusChem. 2022. PMID: 36129761 Free PMC article. Review.
Cited by
-
Evolving ω-amine transaminase AtATA guided by substrate-enzyme binding free energy for enhancing activity and stability against non-natural substrates.Appl Environ Microbiol. 2024 Jul 24;90(7):e0054324. doi: 10.1128/aem.00543-24. Epub 2024 Jun 12. Appl Environ Microbiol. 2024. PMID: 38864627 Free PMC article.
-
Influence of deep eutectic solvents on redox biocatalysis involving alcohol dehydrogenases.Heliyon. 2024 Jun 6;10(12):e32550. doi: 10.1016/j.heliyon.2024.e32550. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38948051 Free PMC article. Review.
-
Cofactor recycling strategies for secondary metabolite production in cell-free protein expression systems.Biophys Rev. 2024 Sep 26;16(5):591-603. doi: 10.1007/s12551-024-01234-1. eCollection 2024 Oct. Biophys Rev. 2024. PMID: 39618802 Free PMC article. Review.
-
Reversible enzyme-catalysed NAD+/NADH electrochemistry.Chem Sci. 2025 Mar 4;16(14):6035-6049. doi: 10.1039/d5sc00570a. eCollection 2025 Apr 2. Chem Sci. 2025. PMID: 40070472 Free PMC article.
References
-
- Berenguer-Murcia A, Fernandez-Lafuente R. 2010. New trends in the recycling of NAD(P)H for the design of sustainable asymmetric reductions catalyzed by dehydrogenases. Curr Org Chem 14:1000–1021. 10.2174/138527210791130514. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

