Tumor Vascular Remodeling Affects Molecular Dissemination to Lymph Node and Systemic Leukocytes
- PMID: 35442085
- PMCID: PMC9508451
- DOI: 10.1089/ten.TEA.2022.0020
Tumor Vascular Remodeling Affects Molecular Dissemination to Lymph Node and Systemic Leukocytes
Abstract
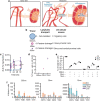
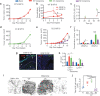
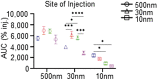
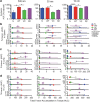
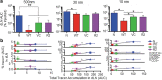
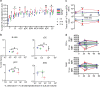
Angiogenic and lymphangiogenic remodeling has long been accepted as a hallmark of cancer development and progression; however, the impacts of this remodeling on immunological responses, which are paramount to the responses to immunotherapeutic treatments, are underexplored. As immunotherapies represent one of the most promising new classes of cancer therapy, in this study, we explore the effects of angiogenic and lymphangiogenic normalization on dissemination of molecules injected into the tumor microenvironment to immune cells in lymph nodes draining the tumor as well as in systemically distributed tissues. A system of fluorescent tracers, size-matched to biomolecules of interest, was implemented to track different mechanisms of tumor transport and access to immune cells. This revealed that the presence of a tumor, and either angiogenic or lymphangiogenic remodeling, altered local retention of model biomolecules, trended toward normalizing dissemination to systemic organs, and modified access to lymph node-resident immune cells in manners dependent on mechanism of transport. More specifically, active cell migration by skin-derived antigen presenting cells was enhanced by both the presence of a tumor and lymphangiogenic normalization, while both angiogenic and lymphangiogenic normalization restored patterns of immune cell access to passively draining species. As a whole, this work uncovers the potential ramifications of tumor-induced angiogenesis and lymphangiogenesis, along with impacts of interrogation into these pathways, on access of tumor-derived species to immune cells. Impact Statement Angiogenic and lymphangiogenic normalization strategies have been utilized clinically to interrogate tumor vasculature with some success. In the age of immunotherapy, the impacts of these therapeutic interventions on immune remodeling are unclear. This work utilizes mouse models of angiogenic and lymphangiogenic normalization, along with a system of fluorescently tagged tracers, to uncover the impacts of angiogenesis and lymphangiogenesis on access of tumor-derived species to immune cell subsets within various organs.
Keywords: angiogenic normalization; biodistribution; growth factor; immune remodeling; lymphangiogenesis; tissue engineering.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Tumor Vasculature as an Emerging Pharmacological Target to Promote Anti-Tumor Immunity.Int J Mol Sci. 2023 Feb 23;24(5):4422. doi: 10.3390/ijms24054422. Int J Mol Sci. 2023. PMID: 36901858 Free PMC article. Review.
-
Angiogenic and lymphangiogenic cascades in the tumor microenvironment.Front Biosci (Schol Ed). 2011 Jan 1;3(1):216-25. doi: 10.2741/s146. Front Biosci (Schol Ed). 2011. PMID: 21196371 Review.
-
Targeting VEGFR-3/-2 signaling pathways with AD0157: a potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases.J Hematol Oncol. 2017 Jun 19;10(1):122. doi: 10.1186/s13045-017-0484-1. J Hematol Oncol. 2017. PMID: 28629427 Free PMC article.
-
Manipulation of the crosstalk between tumor angiogenesis and immunosuppression in the tumor microenvironment: Insight into the combination therapy of anti-angiogenesis and immune checkpoint blockade.Front Immunol. 2022 Nov 10;13:1035323. doi: 10.3389/fimmu.2022.1035323. eCollection 2022. Front Immunol. 2022. PMID: 36439137 Free PMC article. Review.
-
Tumor and lymph node lymphangiogenesis--impact on cancer metastasis.J Leukoc Biol. 2006 Oct;80(4):691-6. doi: 10.1189/jlb.1105653. Epub 2006 Jun 22. J Leukoc Biol. 2006. PMID: 16793912 Review.
Cited by
-
Lymphatic vessel network injury reduces local tumor control despite preservation of the tumor-draining lymph node.Sci Rep. 2025 Jan 28;15(1):3485. doi: 10.1038/s41598-025-85670-3. Sci Rep. 2025. PMID: 39875798 Free PMC article.
-
Consequences of the perivascular niche remodeling for tumoricidal T-cell trafficking into metastasis of ovarian cancer.Res Sq [Preprint]. 2024 Sep 17:rs.3.rs-4940287. doi: 10.21203/rs.3.rs-4940287/v1. Res Sq. 2024. PMID: 39372930 Free PMC article. Preprint.
References
-
- Sung, H., Ferlay, J., Siegel, R.L., et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71, 209, 2021. - PubMed
-
- Howlander, N., Noone, A.M., Krapcho, M., et al. . SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute, 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

