Anion-Exchange Membrane Water Electrolyzers
- PMID: 35442645
- PMCID: PMC9284563
- DOI: 10.1021/acs.chemrev.1c00854
Anion-Exchange Membrane Water Electrolyzers
Abstract
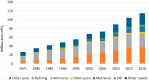
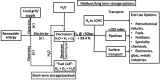
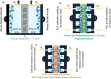
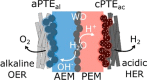
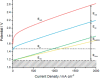
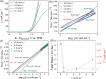
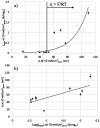
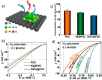
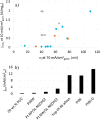
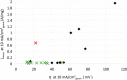
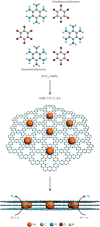
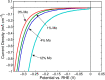
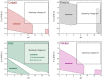
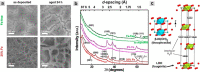
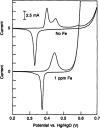
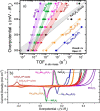
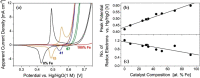
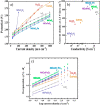
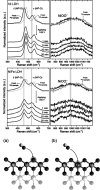
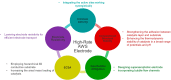
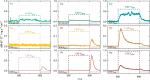
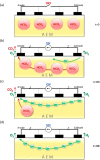
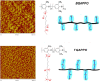
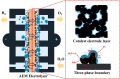
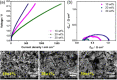
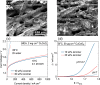
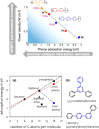
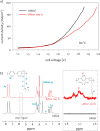
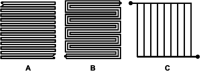
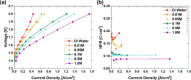
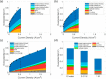
This Review provides an overview of the emerging concepts of catalysts, membranes, and membrane electrode assemblies (MEAs) for water electrolyzers with anion-exchange membranes (AEMs), also known as zero-gap alkaline water electrolyzers. Much of the recent progress is due to improvements in materials chemistry, MEA designs, and optimized operation conditions. Research on anion-exchange polymers (AEPs) has focused on the cationic head/backbone/side-chain structures and key properties such as ionic conductivity and alkaline stability. Several approaches, such as cross-linking, microphase, and organic/inorganic composites, have been proposed to improve the anion-exchange performance and the chemical and mechanical stability of AEMs. Numerous AEMs now exceed values of 0.1 S/cm (at 60-80 °C), although the stability specifically at temperatures exceeding 60 °C needs further enhancement. The oxygen evolution reaction (OER) is still a limiting factor. An analysis of thin-layer OER data suggests that NiFe-type catalysts have the highest activity. There is debate on the active-site mechanism of the NiFe catalysts, and their long-term stability needs to be understood. Addition of Co to NiFe increases the conductivity of these catalysts. The same analysis for the hydrogen evolution reaction (HER) shows carbon-supported Pt to be dominating, although PtNi alloys and clusters of Ni(OH)2 on Pt show competitive activities. Recent advances in forming and embedding well-dispersed Ru nanoparticles on functionalized high-surface-area carbon supports show promising HER activities. However, the stability of these catalysts under actual AEMWE operating conditions needs to be proven. The field is advancing rapidly but could benefit through the adaptation of new in situ techniques, standardized evaluation protocols for AEMWE conditions, and innovative catalyst-structure designs. Nevertheless, single AEM water electrolyzer cells have been operated for several thousand hours at temperatures and current densities as high as 60 °C and 1 A/cm2, respectively.
Conflict of interest statement
The authors declare no competing financial interest.
Figures













References
-
- Connelly E.; Elgowainy A.; Ruth M.. Current Hydrogen Market Size: Domestic and Global; DOE Hydrogen and Fuel Cells Program: 2019; pp 1–5.
-
- The Future of Hydrogen; International Energy Agency: 2019; p 18.
-
- Sample Report: Hydrogen Generation Market Analyses; Grand View Research (CVR), 2020; pp 6–7.
-
- Probstein R. F.; Hicks R. E.. Synthetic Fuels; McGraw-Hill: New York, 1982; p 20.
-
- The engineering toolbox. https://www.engineeringtoolbox.com/ (accessed Oct 2020).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

