Quaternary Ammonium Ion-Tethered (Ambient-Temperature) HDDA Reactions
- PMID: 35442671
- PMCID: PMC9081259
- DOI: 10.1021/jacs.2c00877
Quaternary Ammonium Ion-Tethered (Ambient-Temperature) HDDA Reactions
Abstract
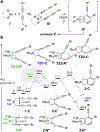
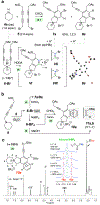
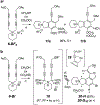
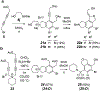
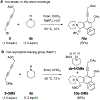
The hexadehydro-Diels-Alder (HDDA) reaction converts a 1,3-diyne bearing a tethered alkyne (the diynophile) into bicyclic benzyne intermediates upon thermal activation. With only a few exceptions, this unimolecular cycloisomerization requires, depending on the nature of the atoms connecting the diyne and diynophile, reaction temperatures of ca. 80-130 °C to achieve a convenient half-life (e.g., 1-10 h) for the reaction. In this report, we divulge a new variant of the HDDA process in which the tether contains a central, quaternized nitrogen atom. This construct significantly lowers the activation barrier for the HDDA cycloisomerization to the benzyne. Moreover, many of the ammonium ion-based, alkyne-containing substrates can be spontaneously assembled, cyclized to benzyne, and trapped in a single-vessel, ambient-temperature operation. DFT calculations provide insights into the origin of the enhanced rate of benzyne formation.
Conflict of interest statement
The authors declare to having no competing interests.
Figures



References
-
- Menschutkin N Beiträge zur Kenntnis der Affinitätskoeffizienten der Alkylhaloide und der organischen Amine. Z. Physik. Chem 1890, 5, 589–600.
- Menschutkin N Über die Affinitätskoeffizienten der Alkylhaloide und der Amine. Z. Physik. Chem 1890, 6, 41–57.
- Smith MB; March J March’s Advanced Organic Chemistry, 6th edition. Wiley, 2007, pp 555–559. ISBN 13: 978-0-471-72091-7.
-
- Hofmann AW Beiträge zur Kenntnifs der flüchtigen organischen Basen. Justus Liebigs Ann. Chem 1851, 79, 11–39.
-
- Liang Y; Hong X; Yu P; Houk KN Why alkynyl substituents dramatically accelerate hexadehydro-Diels-Alder (HDDA) reactions: Stepwise mechanisms of HDDA cycloadditions. Org. Lett 2014, 16, 5702–5705. - PubMed
- Skraba-Joiner SL; Johnson RP; Agarwal J Dehydropericyclic reactions: Symmetry-controlled routes to strained reactive intermediates. J. Org. Chem 2015, 80, 11779–11787. - PubMed
- Marell DJ; Furan LR; Woods BP; Lei X; Bendelsmith AJ; Cramer CJ; Hoye TR; Kuwata KT Mechanism of the intramolecular hexadehydro-Diels−Alder reaction. J. Org. Chem 2015, 80, 11744–11754. - PMC - PubMed
- Wang T; Niu D; Hoye TR The hexadehydro-Diels–Alder (HDDA) cycloisomerization reaction proceeds by a stepwise mechanism. J. Am. Chem. Soc 2016, 138, 7832–7835. - PMC - PubMed
-
- Miyawaki K; Suzuki R; Kawano T; Ueda I Cycloaromatization of a non-conjugated polyenyne system: Synthesis of 5H-benzo[d]fluoreno[3,2-b]pyrans via diradicals generated from1-[2-{4-(2-alkoxymethylphenyl)butan-1,3-diynyl}]phenylpentan-2,4-diyn-l-ols and trapping evidence for the 1,2-didehydrobenzene diradical. Tetrahedron Lett. 1997, 38, 3943–3946.
- Hoye TR; Baire B; Niu D; Willoughby PH; Woods BP The hexadehydro-Diels–Alder reaction. Nature. 2012, 490, 208–212. - PMC - PubMed
- Karmakar R; Lee D Total synthesis of selaginpulvilin C and D relying on in situ formation of arynes and their hydrogenation. Org. Lett 2016, 18, 6105–6107. - PubMed
- Yoshida S; Shimizu K; Uchida K; Hazama Y; Igawa K; Tomooka K; Hosoya T Construction of condensed polycyclic aromatic frameworks through intramolecular cycloaddition reactions involving arynes bearing an internal alkyne moiety. Chem. Eur. J 2017, 23, 15332–15335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

