Chemoenzymatic Synthesis of the Most Pleasant Stereoisomer of Jessemal
- PMID: 35442680
- PMCID: PMC9087343
- DOI: 10.1021/acs.joc.2c00427
Chemoenzymatic Synthesis of the Most Pleasant Stereoisomer of Jessemal
Abstract
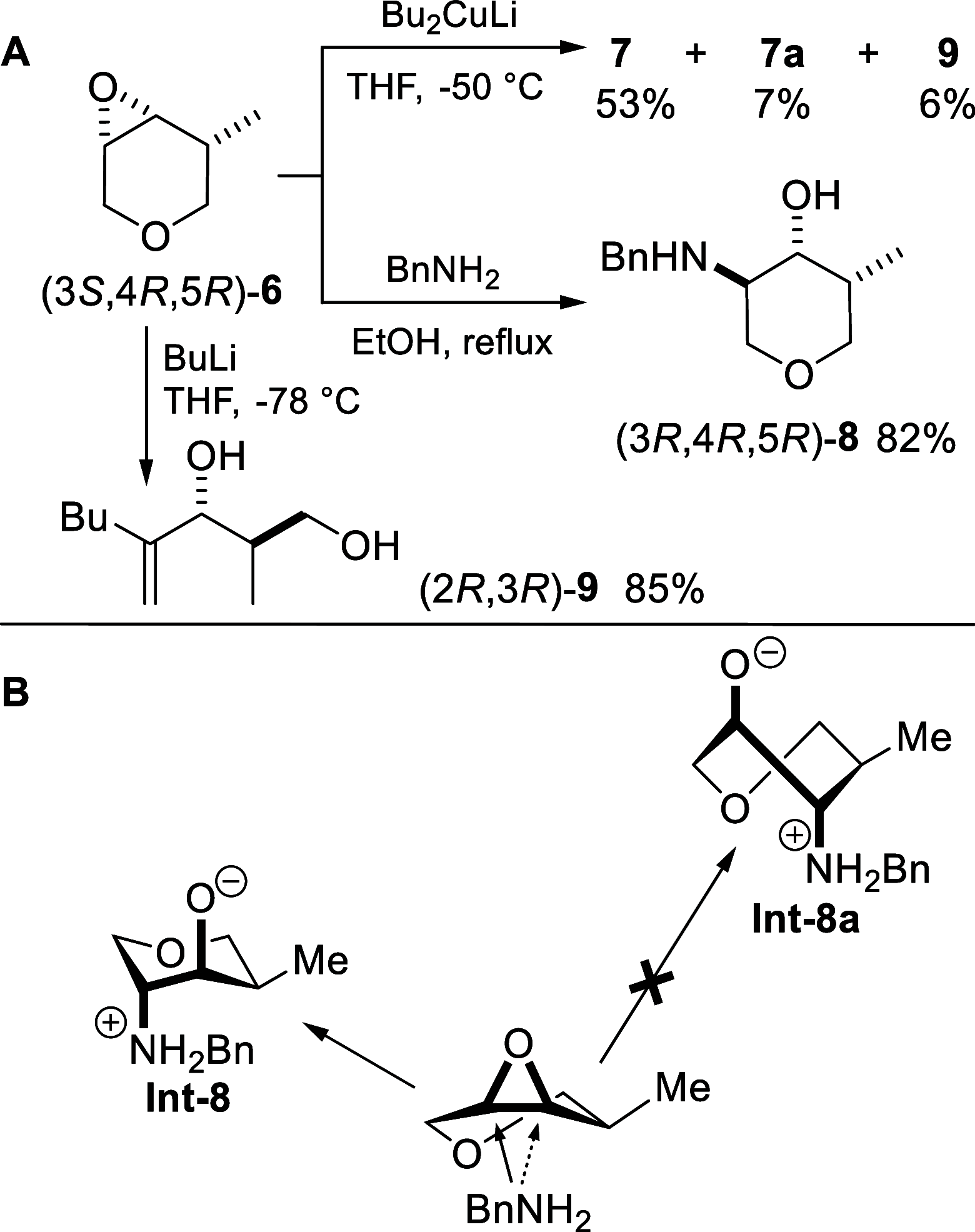
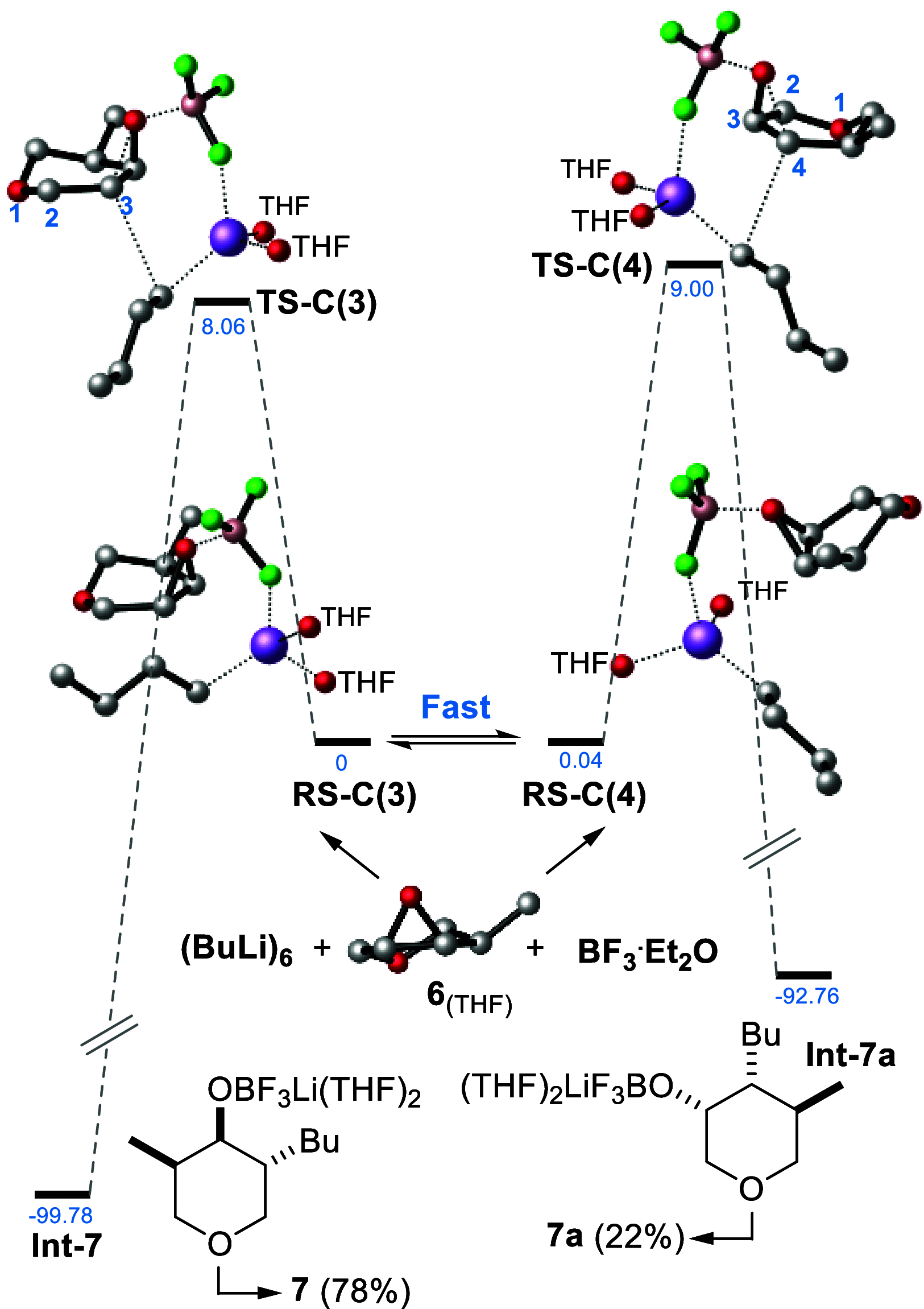
We describe the asymmetric synthesis of the most pleasant enantiomer of Jessemal fragrance. The key steps are (i) the one-pot reduction of an α-chloro-tetrasubstituted cyclohexenone to give the chlorohydrin, catalyzed by two stereoselective redox enzymes (an ene-reductase and an alcohol dehydrogenase); (ii) the regioselective epoxide ring-opening with organocuprate or organolithium nucleophiles. Density functional theory calculations together with the Curtin-Hammett principle allowed the rationalization of the regioselectivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Bentley R. The Nose as a Stereochemist. Enantiomers and Odor. Chem. Rev. 2006, 106, 4099–4112. 10.1021/cr050049t. - DOI - PubMed
- Brenna E.; Fuganti C.; Gatti F. G.; Serra S. Biocatalytic Methods for the Synthesis of Enantioenriched Odor Active Compounds. Chem. Rev. 2011, 111, 4036–4072. 10.1021/cr100289r. - DOI - PubMed
-
- For an example of odorless stereoisomer, see:Brenna E.; Fuganti C.; Gatti F. G.; Malpezzi L.; Serra S. Synthesis and olfactory evaluation of all stereoisomers of the fragrance Nectaryl®. Tetrahedron: Asymmetry 2008, 19, 800–807. 10.1016/j.tetasy.2008.03.011. - DOI
-
- Abate A.; Brenna E.; Fronza G.; Fuganti C.; Gatti F. G.; Maroncelli S. Enzyme-Mediated Preparation of the Enantiomerically Enriched Isomers of the Odorous Tetrahydropyranyl Acetates Jasmal® and Jessemal®, and Their Olfactory Evaluation. Chem. Biodiversity 2006, 3, 677–694. 10.1002/cbdv.200690070. - DOI - PubMed
-
- Venturi S.; Brenna E.; Colombo D.; Fraaije M. W.; Gatti F. G.; Macchi P.; Monti D.; Trajkovic M.; Zamboni E. MultienzymaticStereoselective Reduction of Tetrasubstituted Cyclic Enones to Halohydrins with Three Contiguous Stereogenic Centers. ACS Catal. 2020, 10, 13050–13057. 10.1021/acscatal.0c04097. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

