A somatic mutation in moesin drives progression into acute myeloid leukemia
- PMID: 35442741
- PMCID: PMC9020775
- DOI: 10.1126/sciadv.abm9987
A somatic mutation in moesin drives progression into acute myeloid leukemia
Abstract
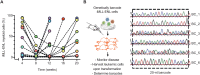
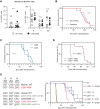
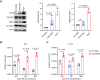
Acute myeloid leukemia (AML) arises when leukemia-initiating cells, defined by a primary genetic lesion, acquire subsequent molecular changes whose cumulative effects bypass tumor suppression. The changes that underlie AML pathogenesis not only provide insights into the biology of transformation but also reveal novel therapeutic opportunities. However, backtracking these events in transformed human AML samples is challenging, if at all possible. Here, we approached this question using a murine in vivo model with an MLL-ENL fusion protein as a primary molecular event. Upon clonal transformation, we identified and extensively verified a recurrent codon-changing mutation (Arg295Cys) in the ERM protein moesin that markedly accelerated leukemogenesis. Human cancer-associated moesin mutations at the conserved arginine-295 residue similarly enhanced MLL-ENL-driven leukemogenesis. Mechanistically, the mutation interrupted the stability of moesin and conferred a neomorphic activity to the protein, which converged on enhanced extracellular signal-regulated kinase activity. Thereby, our studies demonstrate a critical role of ERM proteins in AML, with implications also for human cancer.
Figures



References
-
- Wang J. C., Dick J. E., Cancer stem cells: Lessons from leukemia. Trends Cell Biol. 15, 494–501 (2005). - PubMed
-
- Mitchell K., Steidl U., Targeting immunophenotypic markers on leukemic stem cells: How lessons from current approaches and advances in the leukemia stem cell (LSC) model can inform better strategies for treating acute myeloid leukemia (AML). Cold Spring Harb. Perspect. Med. 10, a036251 (2020). - PMC - PubMed
-
- Krivtsov A. V., Armstrong S. A., MLL translocations, histone modifications and leukaemia stem-cell development. Nat. Rev. Cancer 7, 823–833 (2007). - PubMed
-
- Grossmann V., Schnittger S., Poetzinger F., Kohlmann A., Stiel A., Eder C., Fasan A., Kern W., Haferlach T., Haferlach C., High incidence of RAS signalling pathway mutations in MLL-rearranged acute myeloid leukemia. Leukemia 27, 1933–1936 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

