Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study
- PMID: 35442766
- PMCID: PMC9462531
- DOI: 10.1200/JCO.21.01926
Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study
Erratum in
-
Erratum: Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study.J Clin Oncol. 2024 Feb 1;42(4):486. doi: 10.1200/JCO.23.02629. Epub 2023 Dec 19. J Clin Oncol. 2024. PMID: 38113437 Free PMC article. No abstract available.
Abstract
Purpose: Patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC) have poor prognosis. For these patients, treatment options are limited after first-line systemic therapy.
Patients and methods: In this open-label phase III clinical study, patients with advanced or metastatic ESCC, whose tumor progressed after first-line systemic treatment, were randomly assigned (1:1) to receive intravenous tislelizumab, an anti-programmed cell death protein 1 antibody, 200 mg every 3 weeks or chemotherapy (investigator's choice of paclitaxel, docetaxel, or irinotecan). The primary end point was overall survival (OS) in all patients. The key secondary end point was OS in patients with programmed death-ligand 1 tumor area positivity (TAP) score ≥ 10%.
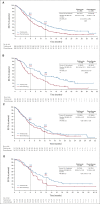
Results: In total, 512 patients across 11 countries/regions were randomly assigned. At final analysis, conducted after 410 death events occurred, OS was significantly longer with tislelizumab versus chemotherapy in all patients (median, 8.6 v 6.3 months; hazard ratio [HR], 0.70 [95% CI, 0.57 to 0.85]; one-sided P = .0001), and in patients with TAP ≥ 10% (median, 10.3 months v 6.8 months; HR, 0.54 [95% CI, 0.36 to 0.79]; one-sided P = .0006). Survival benefit was consistently observed across all predefined subgroups, including those defined by baseline TAP score, region, and race. Treatment with tislelizumab was associated with higher objective response rate (20.3% v 9.8%) and a more durable antitumor response (median, 7.1 months v 4.0 months) versus chemotherapy in all patients. Fewer patients experienced ≥ grade 3 treatment-related adverse events (18.8% v 55.8%) with tislelizumab versus chemotherapy.
Conclusion: Tislelizumab significantly improved OS compared with chemotherapy as second-line therapy in patients with advanced or metastatic ESCC, with a tolerable safety profile. Patients with programmed death-ligand 1 TAP ≥ 10% also demonstrated statistically significant survival benefit with tislelizumab versus chemotherapy.
Trial registration: ClinicalTrials.gov NCT03430843.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 - PubMed
-
- Huang FL, Yu SJ: Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg 41:210-215, 2018 - PubMed
-
- SEER: Cancer Stat Facts: Esophageal Cancer . 2021. https://seer.cancer.gov/statfacts/html/esoph.html
-
- Shah MA, Kennedy EB, Catenacci DV, et al. : Treatment of locally advanced esophageal carcinoma: ASCO Guideline. J Clin Oncol 38:2677-2694, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

