Ultrasound stimulation of the motor cortex during tonic muscle contraction
- PMID: 35442956
- PMCID: PMC9020726
- DOI: 10.1371/journal.pone.0267268
Ultrasound stimulation of the motor cortex during tonic muscle contraction
Abstract
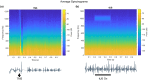
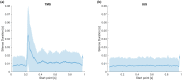
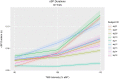
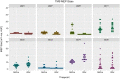
Transcranial ultrasound stimulation (tUS) shows potential as a noninvasive brain stimulation (NIBS) technique, offering increased spatial precision compared to other NIBS techniques. However, its reported effects on primary motor cortex (M1) are limited. We aimed to better understand tUS effects in human M1 by performing tUS of the hand area of M1 (M1hand) during tonic muscle contraction of the index finger. Stimulation during muscle contraction was chosen because of the transcranial magnetic stimulation-induced phenomenon known as cortical silent period (cSP), in which transcranial magnetic stimulation (TMS) of M1hand involuntarily suppresses voluntary motor activity. Since cSP is widely considered an inhibitory phenomenon, it presents an ideal parallel for tUS, which has often been proposed to preferentially influence inhibitory interneurons. Recording electromyography (EMG) of the first dorsal interosseous (FDI) muscle, we investigated effects on muscle activity both during and after tUS. We found no change in FDI EMG activity concurrent with tUS stimulation. Using single-pulse TMS, we found no difference in M1 excitability before versus after sparsely repetitive tUS exposure. Using acoustic simulations in models made from structural MRI of the participants that matched the experimental setups, we estimated in-brain pressures and generated an estimate of cumulative tUS exposure experienced by M1hand for each subject. We were unable to find any correlation between cumulative M1hand exposure and M1 excitability change. We also present data that suggest a TMS-induced MEP always preceded a near-threshold cSP.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

