Ceramide-induced integrated stress response overcomes Bcl-2 inhibitor resistance in acute myeloid leukemia
- PMID: 35443029
- PMCID: PMC9642852
- DOI: 10.1182/blood.2021013277
Ceramide-induced integrated stress response overcomes Bcl-2 inhibitor resistance in acute myeloid leukemia
Abstract
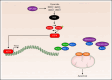
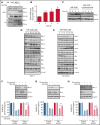
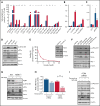
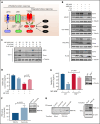
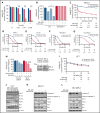
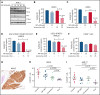
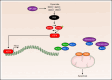
Inducing cell death by the sphingolipid ceramide is a potential anticancer strategy, but the underlying mechanisms remain poorly defined. In this study, triggering an accumulation of ceramide in acute myeloid leukemia (AML) cells by inhibition of sphingosine kinase induced an apoptotic integrated stress response (ISR) through protein kinase R-mediated activation of the master transcription factor ATF4. This effect led to transcription of the BH3-only protein Noxa and degradation of the prosurvival Mcl-1 protein on which AML cells are highly dependent for survival. Targeting this novel ISR pathway, in combination with the Bcl-2 inhibitor venetoclax, synergistically killed primary AML blasts, including those with venetoclax-resistant mutations, as well as immunophenotypic leukemic stem cells, and reduced leukemic engraftment in patient-derived AML xenografts. Collectively, these findings provide mechanistic insight into the anticancer effects of ceramide and preclinical evidence for new approaches to augment Bcl-2 inhibition in the therapy of AML and other cancers with high Mcl-1 dependency.
© 2022 by The American Society of Hematology.
Figures


Comment in
-
Ceramide: improving Bcl-2 inhibitor therapy.Blood. 2022 Jun 30;139(26):3676-3678. doi: 10.1182/blood.2022016608. Blood. 2022. PMID: 35771560 Free PMC article. No abstract available.
References
-
- Morsi RZ, Hage-Sleiman R, Kobeissy H, Dbaibo G. Noxa: role in cancer pathogenesis and treatment. Curr Cancer Drug Targets. 2018;18(10):914-928. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

