Mechanosensation by endothelial PIEZO1 is required for leukocyte diapedesis
- PMID: 35443048
- PMCID: PMC9305087
- DOI: 10.1182/blood.2021014614
Mechanosensation by endothelial PIEZO1 is required for leukocyte diapedesis
Abstract
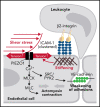
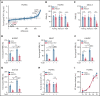
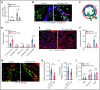
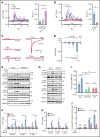
The extravasation of leukocytes is a critical step during inflammation that requires the localized opening of the endothelial barrier. This process is initiated by the close interaction of leukocytes with various adhesion molecules such as ICAM-1 on the surface of endothelial cells. Here we reveal that mechanical forces generated by leukocyte-induced clustering of ICAM-1 synergize with fluid shear stress exerted by the flowing blood to increase endothelial plasma membrane tension and to activate the mechanosensitive cation channel PIEZO1. This leads to increases in [Ca2+]i and activation of downstream signaling events including phosphorylation of tyrosine kinases sarcoma (SRC) and protein tyrosine kinase 2 (PYK2), as well as of myosin light chain, resulting in opening of the endothelial barrier. Mice with endothelium-specific Piezo1 deficiency show decreased leukocyte extravasation in different inflammation models. Thus, leukocytes and the hemodynamic microenvironment synergize to mechanically activate endothelial PIEZO1 and subsequent downstream signaling to initiate leukocyte diapedesis.
© 2022 by The American Society of Hematology.
Figures
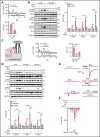
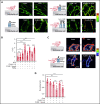
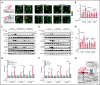
Comment in
-
The tension rises in leukocyte extravasation.Blood. 2022 Jul 21;140(3):165-166. doi: 10.1182/blood.2022016596. Blood. 2022. PMID: 35862093 No abstract available.
References
-
- Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15(11):692-704. - PubMed
-
- Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694-707. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007; 7(9):678-689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

