Obesity-induced elevated palmitic acid promotes inflammation and glucose metabolism disorders through GPRs/NF-κB/KLF7 pathway
- PMID: 35443706
- PMCID: PMC9021212
- DOI: 10.1038/s41387-022-00202-6
Obesity-induced elevated palmitic acid promotes inflammation and glucose metabolism disorders through GPRs/NF-κB/KLF7 pathway
Abstract
Objective: Our previous results have shown that obesity-induced excessive palmitic acid (PA) can promote the expression of KLF7, which plays a vital role in regulation of inflammation, glucose metabolism. But the exact mechanism of PA up-regulating the expression of KLF7 is not clear yet. This study is intend to explore whether PA promoting KLF7 expression through GPRs/NF-κB signaling pathway, causing inflammation and glucose metabolism disorders.
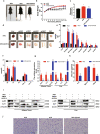
Methods: Cells were blocked GPRs/NF-κB under PA stimulation in vitro to demonstrate the molecular mechanism of PA up-regulates KLF7 expression. The regulatory effect of p65 on KLF7 was detected by luciferase reporter gene assay. Blocking GPRs/NF-κB in diet-induced obesity mice to detect the expression of KLF7, inflammatory cytokines and glucose metabolism related factors, clarifying the effects of GPRs/NF-κB on KLF7 in vivo.
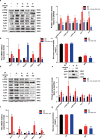
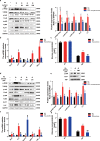
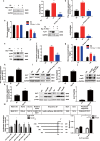
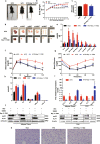
Results: In 3T3-L1 adipocytes and HepG2 cells, PA could up-regulate the expression of KLF7 by promoting the GPR40/120-NF-κB signaling pathway, leading to inflammation and reduced glucose consumption (p < 0.05 for both). Luciferase reporter gene assay and ChIP assay showed that p65 could transcriptionally up-regulates the expression of KLF7. In high-fat diet (HFD) mice, after intraperitoneal injection of GPR40 or GPR120 blocker, the levels of p-p65 and KLF7 in epididymal white adipose tissue and liver were significantly decreased (p < 0.05 for both). Pharmacological inhibition of p-p65 significantly attenuated KLF7 expression and improved glucose tolerant and insulin sensitive (p < 0.05 for both).
Conclusions: Our results indicate that obesity-induced elevated palmitic acid promotes inflammation and glucose metabolism disorders through GPRs/NF-κB/KLF7 signaling pathway.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

