Artesunate improves venetoclax plus cytarabine AML cell targeting by regulating the Noxa/Bim/Mcl-1/p-Chk1 axis
- PMID: 35443722
- PMCID: PMC9021233
- DOI: 10.1038/s41419-022-04810-z
Artesunate improves venetoclax plus cytarabine AML cell targeting by regulating the Noxa/Bim/Mcl-1/p-Chk1 axis
Abstract
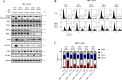
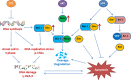
Venetoclax plus cytarabine therapy is approved for elderly acute myeloid leukemia (AML) patients and needs further improvement. We studied the mechanisms of venetoclax plus cytarabine treatment and searched for a third agent to enhance their effects. Cytarabine induces S phase arrest-mediated DNA damage with activation of DNA replication checkpoint kinase 1 (Chk1) through phosphorylation, while venetoclax induces B cell lymphoma 2 (Bcl-2)-interacting mediator of cell death (Bim)-mediated apoptotic DNA damage. Myeloid cell leukemia-1 (Mcl-1) plays negative roles in both events by sequestering Bim and accelerating Chk1 phosphorylation. Venetoclax releases Bim from Bcl-2 with increased Bim binding to Mcl-1. Artesunate, an antimalaria drug, induces Noxa to replace Bim from Mcl-1 and induces synergistic apoptosis with venetoclax accompanied with Mcl-1 reduction. Silencing Mcl-1 or adding venetoclax/artesunate diminishes the cytarabine resistance pathway p-Chk1. The triple combination exhibits S phase arrest with enhanced DNA damage, improves AML colony formation inhibition, and prolongs survival of two mice xenograft models compared to the venetoclax/cytarabine dual combination. Artesunate serves as a bridge for venetoclax and cytarabine combination by Noxa and Bim-mediated apoptosis and Mcl-1 reduction. We provide a new triple combination for AML treatment by targeting the Noxa/Mcl-1/Bim axis to reverse Mcl-1/p-Chk1 resistance of cytarabine therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Short NJ, Konopleva M, Kadia TM, Borthakur G, Ravandi F, DiNardo CD, et al. Advances in the treatment of acute myeloid Leukemia: new drugs and new challenges. Cancer Discov. 2020;10:506–25. doi: 10.1158/2159-8290.CD-19-1011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

