Influence of Acidic Environment on Hydrolytic Stability of MDP-Ca Salts with Nanolayered and Amorphous Structures
- PMID: 35444417
- PMCID: PMC9014115
- DOI: 10.2147/IJN.S357823
Influence of Acidic Environment on Hydrolytic Stability of MDP-Ca Salts with Nanolayered and Amorphous Structures
Abstract
Purpose: This study aimed to investigate the hydrolytic stability of 10-methacryloyloxydecyl dihydrogen phosphate calcium (MDP-Ca) salts with nanolayered and amorphous structures in different pH environments.
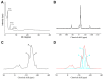
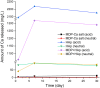
Methods: The MDP-Ca salts were synthesized from MDP and calcium chloride and characterized by X-ray diffraction (XRD), nuclear magnetic resonance (NMR), and transmission electron microscopy (TEM). Inductively coupled plasma-mass spectrometry (ICP-MS) was used to quantify the release of calcium from the synthesized MDP-Ca salt, MDP-treated hydroxyapatite (MDP-HAp), and untreated HAp after soaking in acidic and neutral solutions for 1, 7, and 30 days. To study the hydrolytic process, we carried out molecular dynamics (MD) simulations of the nanolayered MCS-MD (monocalcium salt of the MDP dimer) and DCS-MD (dicalcium salt of the MDP dimer) structures, as well as of the amorphous-phase MCS-MM (monocalcium salt of the MDP monomer).
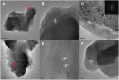
Results: The TEM images showed that the nanolayered structures were partially degraded by acid attack. Based on the ICP-MS results, the hydrolysis rate of the MDP-Ca salt in acidic and neutral environments followed the order HAp > MDP-HAp > MDP-Ca salt. The MD simulations showed that, in acidic environments, clusters of MDP remained aggregated and all Ca2+ ions separated from the MDP monomer to interact with water molecules in aqueous solution. In neutral environments, Ca2+ ions always interacted with phosphate groups, OH- ions, and water molecules to form clusters centered on Ca2+ ions.
Conclusion: MDP-Ca presented higher hydrolysis rates in acidic than neutral environments. Nanolayered MCS-MD possessed the highest resistance to acidic hydrolysis, followed by amorphous MCS-MM and DCS-MD.
Keywords: 10-methacryloyloxydecyl dihydrogen phosphate calcium salts; dentin bonding; hydrolysis; molecular dynamics simulations; nanolayering.
© 2022 Zhao et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

