Nanoscale Diamond-Based Formulation as an Immunomodulator and Potential Therapeutic for Lymphoma
- PMID: 35444547
- PMCID: PMC9014173
- DOI: 10.3389/fphar.2022.852065
Nanoscale Diamond-Based Formulation as an Immunomodulator and Potential Therapeutic for Lymphoma
Abstract
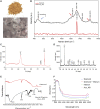
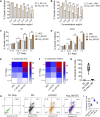
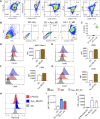
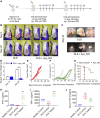
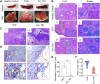
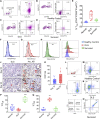
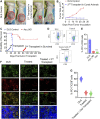
Integrative medicine practices, such as Ayurveda, are popular in India and many South Asian countries, yet basic research to investigate the concepts, procedures, and medical benefits of ayurvedic products has received little attention and is not fully understood. Here, we report a functional nanodiamond-based traditional Ayurvedic herbomineral formulation, Heerak Bhasma (Ayu_ND), for the treatment of solid tumors called Dalton's lymphoma generated in CD1 mice. Ayu_ND-mediated immunostimulation significantly reduces tumor cell proliferation and induces apoptosis aided by the active participation of dendritic cells. Immunomodulatory Ayu_ND treatment is highly immunostimulatory and drives dendritic cells to produce TNF-α. Treatment with Ayu_ND significantly reduces the tumor volume, inhibits metastasis in distant vascularized organs, and increases the life span of tumor-bearing animals compared with untreated littermates. These events were associated with elevated serum levels of the protective cytokines IFN-γ and TNF-α and downregulated the disease, exacerbating TGF-β. Ayu_ND-mediated therapeutic success was also accompanied by the depletion of regulatory T cells and enhanced vaccine-induced T-cell immunity, guided by the restoration of the memory CD8+ T-cell pool and prevention of PD-1-mediated T cell exhaustion. The results provide a basis for further evaluation of ayurvedic formulations and drug efficacy in treating cancers.
Keywords: Heerak Bhasma; T cell; ayurveda; dendritic cell; lymphoma; nanodiamond.
Copyright © 2022 Paladhi, Rej, Sarkar, Singh, Bhattacharyya, Sarkar, Kar, Manna and Hira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bondar’ V. S., Pozdnyakova I. O., Puzyr’ A. P. (2004). Applications of Nanodiamonds for Separation and Purification of Proteins. Phys. Solid State. 46, 758–760. 10.1134/1.1711468 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

