Clinical Implications of Atrial Fibrillation Detection Using Wearable Devices in Patients With Cryptogenic Stroke (CANDLE-AF) Trial: Design and Rationale
- PMID: 35445088
- PMCID: PMC9013795
- DOI: 10.3389/fcvm.2022.837958
Clinical Implications of Atrial Fibrillation Detection Using Wearable Devices in Patients With Cryptogenic Stroke (CANDLE-AF) Trial: Design and Rationale
Erratum in
-
Corrigendum: Clinical implications of atrial fibrillatioN detection using wearabLE devices in patients with cryptogenic stroke (CANDLE-AF) trial: Design and rationale.Front Cardiovasc Med. 2023 Apr 19;10:1199185. doi: 10.3389/fcvm.2023.1199185. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37153467 Free PMC article.
Abstract
Background: Although many electrocardiography wearable devices have been released recently for the detection of atrial fibrillation (AF), there are few studies reporting prospective data for wearable devices compared to the strategy of the existing guidelines in the detection of atrial fibrillation (AF) after cryptogenic stroke. A tiny single-patch monitor is more convenient than a conventional Holter monitor recording device and, therefore, longer duration of monitoring may be acceptable.
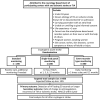
Methods and design: The CANDLE-AF study is a multicenter, prospective, randomized controlled trial. Patients with transient ischemic attack or ischemic stroke without any history of AF will be enrolled. The superiority of the 72-h single-patch monitor to standard strategy and non-inferiority of the 72-h single-patch monitor to an event-recorder-type device will be investigated. Single-patch monitor arm will repeat monitoring at 1, 3, 6, and 12 months, event-recorder-type arm will repeat monitoring twice daily for 12 months. The enrollment goal is a total of 600 patients, and the primary outcome is the detection of AF which continues at least 30 s during study period. The secondary outcome is the rate of changes from antiplatelet to anticoagulant and major adverse cardiac and cerebrovascular events within 1 year.
Conclusions: The results of CANDLE-AF will clarify the role of a single-lead patch ECG for the early detection of AF in patients with acute ischemic stroke. In addition, the secondary outcome will be analyzed to determine whether more sensitive AF detection can affect the prognosis and if further device development is meaningful. (cris.nih.go.kr KCT0005592).
Keywords: atrial fibrillation; cryptogenic stroke; ischemic stroke; rhythm monitoring; single-lead ECG; wearable device.
Copyright © 2022 Jung, Lee, Kang, Shin, Chang, Woo Shin, Park, Kim, Nam, Heo, Kim, Yu, Lee, Heo, Woo, Park, Roh, Kim, Lee, Do, Kim, Song, Park and CANDLE-AF Trial Investigators.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. 10.1161/STR.0000000000000211 - DOI - PubMed
-
- Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. . 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. 10.1161/STR.0000000000000375 - DOI - PubMed
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2021) 42:373–498. 10.1093/eurheartj/ehaa945 - DOI - PubMed
-
- Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RA, et al. . Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol. (2016) 9:e003333. 10.1161/CIRCEP.115.003333 - DOI - PubMed
LinkOut - more resources
Full Text Sources

