Catabolic profiling of selective enzymes in the saccharification of non-food lignocellulose parts of biomass into functional edible sugars and bioenergy: An in silico bioprospecting
- PMID: 35445120
- PMCID: PMC8985887
- DOI: 10.5455/javar.2022.i565
Catabolic profiling of selective enzymes in the saccharification of non-food lignocellulose parts of biomass into functional edible sugars and bioenergy: An in silico bioprospecting
Abstract
Objectives: The research aims to analyze the catabolic strength of different hydrolytic enzymes in assessing the biological conversion potential of lignocellulose parts of agricultural biomass wastes into functional edible sugars and biofuels.
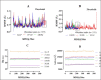
Materials and methods: The enzymes' hydrolytic properties-versatile peroxidase, manganese peroxidase, and lignin peroxidase were used to identify their complexing strength with the lignin substrate, whereas endoglucanase cel12A, acidocaldarius cellulase, and Melanocarpus albomyces endoglucanase were tested on the cellulose gel substrate. Because the biodegradation properties are heavily influenced by the "enzyme-substrate complexing energy level," proper molecular optimization and energy minimization of the enzymes and substrates were carried out, as well as the identification of the enzyme's active sites prior to complexing.comprehensive molecular dynamic simulation was run to study their-alpha carbon, root-mean-square deviation (Å), molecular surface area (Å2), root-mean-square fluctuation (Å), radius of gyration (nm), hydrogen bonds with hydrophobic interactions, and solvent accessible surface area (Å2) values for 50 ns. The simulated data mining was conducted using advanced programming algorithms to establish the final enzyme-substrate complexing strength in binding and catalysis.
Results: Among the lignin-degrading enzymes, versatile peroxidase shows promising catalytic activity with the best docking pose and significant values in all the dynamic simulation parameters. Similarly, Melanocarpus albomyces endoglucanase shows the best activity in all aspects of molecular docking and dynamics among the cellulose-degrading enzymes.
Conclusion: The lignin content of biomass wastes can be degraded into cellulose and hemicellulose using lignin-degrading enzymes. The cellulose can be further degraded into glucose and xylose sugars following the cellulose-degrading enzyme activity. These sugars can be further degraded into biofuel through anaerobic fermentation. Systematic bioconversion of the lignocellulosic components can ensure sustainable biomass management, creating an alternative food and energy source for human beings to face the challenges of global hunger where the enzymes can pave the way.
Keywords: Catabolic profiling; biofuel; enzymatic hydrolysis; functional edible sugars; lignocellulose biomass; molecular dynamic simulation; saccharification.
Copyright: © Journal of Advanced Veterinary and Animal Research.
Conflict of interest statement
The authors have no competing interest at all with the others.
Figures




References
-
- Roser M, Ritchie H, Ortiz-Ospina E. World population growth. Our World in Data. 2020. Available via https://ourworldindata.org/world-population-growth.
-
- Wolff JL, Spillman BC, Freedman VA, Kasper JD. A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Intern Med. 2016;176(3):372–9. https://doi.org/10.1001/jamainternmed.2015.7664. - PMC - PubMed
-
- Masa R, Khan Z, Chowa G. Youth food insecurity in Ghana and South Africa: prevalence, socioeconomic correlates, and moderation effect of gender. Child Youth Serv Rev. 2020;116:105180. https://doi.org/10.1016/j.childyouth.2020.105180.
-
- Zoghlami A, Paës G. Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis. Front Chem. 2019;7:874. https://doi.org/10.3389/fchem.2019.00874. - PMC - PubMed
-
- Abril M, Bastias E, von Schiller D, Martí E, Menéndez M, Muñoz , I. Uptake and trophic transfer of nitrogen and carbon in a temperate forested headwater stream. Aquat Sci. 2019;81(4):1–15. https://doi.org/10.1007/s00027-019-0672-x.
LinkOut - more resources
Full Text Sources
Miscellaneous
