Tregs Attenuate Peripheral Oxidative Stress and Acute Phase Proteins in ALS
- PMID: 35445431
- PMCID: PMC9545429
- DOI: 10.1002/ana.26375
Tregs Attenuate Peripheral Oxidative Stress and Acute Phase Proteins in ALS
Abstract
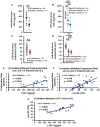
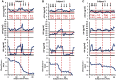
Oxidative stress (OS) induces inflammation, which in turn exacerbates OS and the expression of acute phase proteins (APPs). Regulatory T lymphocyte (Treg) therapy was assessed for suppression of OS and APP responses in longitudinal serum samples from subjects with amyotrophic lateral sclerosis (ALS) enrolled in a phase I clinical trial. The first round of Treg therapy suppressed levels of oxidized low-density lipoprotein (ox-LDL). During a 6-month washout period, ox-LDL levels increased. A second round of therapy again suppressed ox-LDL levels and then rose following the cessation of treatment. Serum levels of APPs, soluble CD14, lipopolysaccharide binding protein, and C-reactive protein, were stabilized during Treg administrations, but rose during the washout period and again after therapy was discontinued. Treg therapy potentially suppresses peripheral OS and the accompanying circulating pro-inflammatory induced APPs, both of which may serve as peripheral candidates for monitoring efficacies of immunomodulating therapies. ANN NEUROL 2022;92:195-200.
© 2022 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
DRB declares a conflict of interest as a consultant with Implicit Bioscience and Coya Therapeutics, Inc. ADT declares a conflict of interest as a consultant with Coya Therapeutics, Inc. SHA declares a conflict of interest as a consultant with Implicit Bioscience and scientific advisory board chair of Coya Therapeutics, Inc. The remaining authors have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

