Signaling pathways and therapeutic approaches in glioblastoma multiforme (Review)
- PMID: 35445737
- PMCID: PMC9084550
- DOI: 10.3892/ijo.2022.5359
Signaling pathways and therapeutic approaches in glioblastoma multiforme (Review)
Abstract
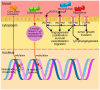
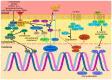
Glioblastoma multiforme (GBM) is the most aggressive type of primary brain tumor and is associated with a poor clinical prognosis. Despite the progress in the understanding of the molecular and genetic changes that promote tumorigenesis, effective treatment options are limited. The present review intended to identify and summarize major signaling pathways and genetic abnormalities involved in the pathogenesis of GBM, as well as therapies that target these pathways. Glioblastoma remains a difficult to treat tumor; however, in the last two decades, significant improvements in the understanding of GBM biology have enabled advances in available therapeutics. Significant genomic events and signaling pathway disruptions (NF‑κB, Wnt, PI3K/AKT/mTOR) involved in the formation of GBM were discussed. Current therapeutic options may only marginally prolong survival and the current standard of therapy cures only a small fraction of patients. As a result, there is an unmet requirement for further study into the processes of glioblastoma pathogenesis and the discovery of novel therapeutic targets in novel signaling pathways implicated in the evolution of glioblastoma.
Keywords: glioblastoma; immunotherapy; mutations; signaling pathways; targeted therapy; tumor treating fields.
Conflict of interest statement
YB has been a speaker for Amgen and Regeneron and has been an Advisor/Board Member for Alphageneron, G1 Therapeutics and Jazz Pharmaceuticals. The remaining authors have no competing interests to declare.
Figures
References
-
- Faleh TA, Juweid M. Epidemiology and outcome of glioblastoma. Exon Publications. 2017:143–153. - PubMed
-
- World Health Organization . Histological classification of tumors of the central nervous system. Lyon, France: IARC; 2016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
