Molecular Pathogenesis of Sporadic Desmoid Tumours and Its Implications for Novel Therapies: A Systematised Narrative Review
- PMID: 35446005
- PMCID: PMC9217905
- DOI: 10.1007/s11523-022-00876-z
Molecular Pathogenesis of Sporadic Desmoid Tumours and Its Implications for Novel Therapies: A Systematised Narrative Review
Abstract
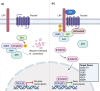
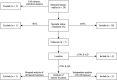
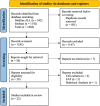
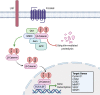
Sporadic desmoid-type fibromatosis is a rare, fibroblastic soft-tissue neoplasm with local aggressiveness but no metastatic potential. Aberrant Wnt/β-catenin signalling has been extensively linked to desmoid pathogenesis, although little is known about other molecular drivers and no established treatment approach exists. We aimed to summarise the current literature regarding the molecular pathogenesis of sporadic desmoid-type fibromatosis and to discuss the effects of both current and emerging novel therapies targeting these mechanisms. A literature search was conducted of MEDLINE® ALL and EMBASE databases for published studies (2000-August 2021) using keywords related to 'fibromatosis aggressive', 'immunohistochemistry', 'polymerase chain reaction' and 'mutation'. Articles were included if they examined the role of proteins in sporadic or extra-abdominal human desmoid-type fibromatosis pathogenesis. Searching identified 1684 articles. Following duplicate removal and eligibility screening, 36 were identified. After a full-text screen, 22 were included in the final review. At least 47% of desmoid-type fibromatosis cases displayed aberrant β-catenin immunoreactivity amongst ten studies. Cyclin D1 overexpression occurred in at least 40% of cases across five studies. Six studies reported oestrogen receptor-β expression with a range of 7.4-90%. Three studies implicated matrix metalloproteinases, with one study demonstrating vascular endothelial growth factor overexpression. One study explored the positive relationship between cyclooxygenase-2 and platelet-derived growth factor receptor-β. Aberrant Wnt/β-catenin signalling is a well-established pathogenic driver that may be targeted via downstream modulation. Growth factor signalling is best appreciated through the clinical trial effects of multi-targeted tyrosine kinase inhibitors, whilst oestrogen receptor expression data may only offer a superficial insight into oestrogen signalling. Finally, the tumour microenvironment presents multiple potential novel therapeutic targets.
Plain language summary
Sporadic desmoid tumours are rare soft-tissue neoplasms that arise from connective tissues in the chest wall, head, neck and limbs. Whilst lacking metastatic potential, uncertainty surrounding their locally aggressive growth and unpredictable recurrence complicates treatment approaches. At the molecular level, alterations in the Wnt/β-catenin signalling pathway, a fundamental coordinator of cell growth and development, have been strongly linked to desmoid tumour development. Beyond this, however, little is known about other molecular drivers. In the case of progressive or life-threatening disease, complex treatment decisions are made regarding the use of surgery, radiotherapy or systemic treatment modalities. Of the targeted systemic therapies, a lack of comparative clinical studies further complicates medical treatment decision making as no definitive treatment approach exists. Therefore, this review aimed to summarise the literature regarding the molecular drivers of desmoid tumour pathogenesis and to discuss the current and emerging novel therapies targeting such mechanisms. Utilising findings from human desmoid tissue samples, we present the rationale for targeting downstream mediators of the central Wnt/β-catenin pathway and outline potential treatment targets in the tumour microenvironment. We also highlight the knowledge gained from clinical drug trials targeting desmoid growth factor signalling and present the potentially superficial insight provided by oestrogen receptor expression profiles on the role of oestrogen signalling in desmoid pathogenesis. In doing so, this work may assist in the eventual development of an evidence-based treatment approach for sporadic desmoid tumours.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures


References
-
- Fletcher CDM. WHO classification of tumours of soft tissue and bone. World Health Organization classification of tumours. Geneva: International Agency for Research on Cancer; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

