Interbacterial Antagonism Mediated by a Released Polysaccharide
- PMID: 35446119
- PMCID: PMC9112932
- DOI: 10.1128/jb.00076-22
Interbacterial Antagonism Mediated by a Released Polysaccharide
Abstract
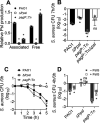
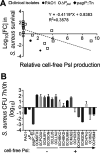
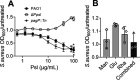
Pseudomonas aeruginosa and Staphylococcus aureus are two common pathogens causing chronic infections in the lungs of people with cystic fibrosis (CF) and in wounds, suggesting that these two organisms coexist in vivo. However, P. aeruginosa utilizes various mechanisms to antagonize S. aureus when these organisms are grown together in vitro. Here, we suggest a novel role for Psl in antagonizing S. aureus growth. Psl is an exopolysaccharide that exists in both cell-associated and cell-free forms and is important for biofilm formation in P. aeruginosa. When grown in planktonic coculture with a P. aeruginosa psl mutant, S. aureus had increased survival compared to when it was grown with wild-type P. aeruginosa. We found that cell-free Psl was critical for the killing, as purified cell-free Psl was sufficient to kill S. aureus. Transmission electron microscopy of S. aureus treated with Psl revealed disrupted cell envelopes, suggesting that Psl causes S. aureus cell lysis. This was independent of known mechanisms used by P. aeruginosa to antagonize S. aureus. Cell-free Psl could also promote S. aureus killing during growth in in vivo-like conditions. We also found that Psl production in P. aeruginosa CF clinical isolates positively correlated with the ability to kill S. aureus. This could be a result of P. aeruginosa coevolution with S. aureus in CF lungs. In conclusion, this study defines a novel role for P. aeruginosa Psl in killing S. aureus, potentially impacting the coexistence of these two opportunistic pathogens in vivo. IMPORTANCE Pseudomonas aeruginosa and Staphylococcus aureus are two important opportunistic human pathogens commonly coisolated from clinical samples. However, P. aeruginosa can utilize various mechanisms to antagonize S. aureus in vitro. Here, we investigated the interactions between these two organisms and report a novel role for P. aeruginosa exopolysaccharide Psl in killing S. aureus. We found that cell-free Psl could kill S. aureus in vitro, possibly by inducing cell lysis. This was also observed in conditions reflective of in vivo scenarios. In accord with this, Psl production in P. aeruginosa clinical isolates positively correlated with their ability to kill S. aureus. Together, our data suggest a role for Psl in affecting the coexistence of P. aeruginosa and S. aureus in vivo.
Keywords: Pseudomonas aeruginosa; Psl; Staphylococcus aureus; cell lysis; cystic fibrosis; exopolysaccharide; polymicrobial; wound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Exogenous Alginate Protects Staphylococcus aureus from Killing by Pseudomonas aeruginosa.J Bacteriol. 2020 Mar 26;202(8):e00559-19. doi: 10.1128/JB.00559-19. Print 2020 Mar 26. J Bacteriol. 2020. PMID: 31792010 Free PMC article.
-
Pseudomonas aeruginosa Alginate Overproduction Promotes Coexistence with Staphylococcus aureus in a Model of Cystic Fibrosis Respiratory Infection.mBio. 2017 Mar 21;8(2):e00186-17. doi: 10.1128/mBio.00186-17. mBio. 2017. PMID: 28325763 Free PMC article.
-
Matrix Polysaccharides and SiaD Diguanylate Cyclase Alter Community Structure and Competitiveness of Pseudomonas aeruginosa during Dual-Species Biofilm Development with Staphylococcus aureus.mBio. 2018 Nov 6;9(6):e00585-18. doi: 10.1128/mBio.00585-18. mBio. 2018. PMID: 30401769 Free PMC article.
-
Molecular Mechanisms of Staphylococcus and Pseudomonas Interactions in Cystic Fibrosis.Front Cell Infect Microbiol. 2022 Jan 6;11:824042. doi: 10.3389/fcimb.2021.824042. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35071057 Free PMC article. Review.
-
Friends or enemies? The complicated relationship between Pseudomonas aeruginosa and Staphylococcus aureus.Mol Microbiol. 2021 Jul;116(1):1-15. doi: 10.1111/mmi.14699. Epub 2021 Mar 8. Mol Microbiol. 2021. PMID: 33576132 Review.
Cited by
-
A bacterial pigment provides cross-species protection from H2O2- and neutrophil-mediated killing.Proc Natl Acad Sci U S A. 2024 Jan 9;121(2):e2312334121. doi: 10.1073/pnas.2312334121. Epub 2024 Jan 3. Proc Natl Acad Sci U S A. 2024. PMID: 38170744 Free PMC article.
-
Pseudomonas aeruginosa biofilm exopolysaccharides: assembly, function, and degradation.FEMS Microbiol Rev. 2023 Nov 1;47(6):fuad060. doi: 10.1093/femsre/fuad060. FEMS Microbiol Rev. 2023. PMID: 37884397 Free PMC article. Review.
-
Synthesis of the Pentasaccharide Unit of the Pseudomonas aeruginosa Exopolysaccharide Psl Conjugation with CRM197, and Evaluation of Antigenicity in a QS-21/Pam3CSK4-Liposomal Formulation.Molecules. 2025 Apr 11;30(8):1720. doi: 10.3390/molecules30081720. Molecules. 2025. PMID: 40333663 Free PMC article.
-
Pathogen-pathogen interactions during co-infections.ISME J. 2025 Jan 2;19(1):wraf104. doi: 10.1093/ismejo/wraf104. ISME J. 2025. PMID: 40407166 Free PMC article. Review.
-
Stenotrophomonas maltophilia exhibits defensive multicellularity in response to a Pseudomonas aeruginosa quorum sensing molecule.bioRxiv [Preprint]. 2025 May 2:2025.05.02.651457. doi: 10.1101/2025.05.02.651457. bioRxiv. 2025. PMID: 40568125 Free PMC article. Preprint.
References
-
- Wakeman CA, Moore JL, Noto MJ, Zhang Y, Singleton MD, Prentice BM, Gilston BA, Doster RS, Gaddy JA, Chazin WJ, Caprioli RM, Skaar EP. 2016. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat Commun 7:11951. 10.1038/ncomms11951. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

