Identifying Patients Whose Symptoms Are Underrecognized During Treatment With Breast Radiotherapy
- PMID: 35446337
- PMCID: PMC9026246
- DOI: 10.1001/jamaoncol.2022.0114
Identifying Patients Whose Symptoms Are Underrecognized During Treatment With Breast Radiotherapy
Abstract
Importance: Understanding whether physicians accurately detect symptoms in patients with breast cancer is important because recognition of symptoms facilitates supportive care, and clinical trials often rely on physician assessments using Common Toxicity Criteria for Adverse Events (CTCAE).
Objective: To compare the patient-reported outcomes (PROs) of patients with breast cancer who received radiotherapy from January 1, 2012, to March 31, 2020, with physicians' CTCAE assessments to assess underrecognition of symptoms.
Design, setting, and participants: This cohort study included a total of 29 practices enrolled in the Michigan Radiation Oncology Quality Consortium quality initiative. Of 13 725 patients with breast cancer who received treatment with radiotherapy after undergoing lumpectomy, 9941 patients (72.4%) completed at least 1 PRO questionnaire during treatment with radiotherapy and were evaluated for the study. Of these, 9868 patients (99.3%) were matched to physician CTCAE assessments that were completed within 3 days of the PRO questionnaires.
Exposures: Patient and physician ratings of 4 symptoms (pain, pruritus, edema, and fatigue) were compared.
Main outcomes and measures: We used multilevel multivariable logistic regression to evaluate factors associated with symptom underrecognition, hypothesizing that it would be more common in racial and ethnic minority groups.
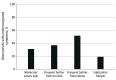
Results: Of 9941 patients, all were female, 1655 (16.6%) were Black, 7925 (79.7%) were White, and 361 (3.6%) had Other race and ethnicity (including American Indian/Alaska Native, Arab/Middle Eastern, and Asian), either as self-reported or as indicated in the electronic medical record. A total of 1595 (16.0%) were younger than 50 years, 2874 (28.9%) were age 50 to 59 years, 3353 (33.7%) were age 60 to 69 years, and 2119 (21.3%) were 70 years or older. Underrecognition of symptoms existed in 2094 of 6781 (30.9%) observations of patient-reported moderate/severe pain, 748 of 2039 observations (36.7%) of patient-reported frequent pruritus, 2309 of 4492 observations (51.4%) of patient-reported frequent edema, and 390 of 2079 observations (18.8%) of patient-reported substantial fatigue. Underrecognition of at least 1 symptom occurred at least once for 2933 of 5510 (53.2%) of those who reported at least 1 substantial symptom. Factors independently associated with underrecognition were younger age (younger than 50 years compared with 60-69 years: odds ratio [OR], 1.35; 95% CI, 1.14-1.59; P < .001; age 50-59 years compared with 60-69 years: OR, 1.19; 95% CI, 1.03-1.37; P = .02), race (Black individuals compared with White individuals: OR, 1.56; 95% CI 1.30-1.88; P < .001; individuals with Other race or ethnicity compared with White individuals: OR, 1.52; 95% CI, 1.12-2.07; P = .01), conventional fractionation (OR, 1.26; 95% CI, 1.10-1.45; P = .002), male physician sex (OR, 1.54; 95% CI, 1.20-1.99; P = .002), and 2-field radiotherapy (without a supraclavicular field) (OR, 0.80; 95% CI, 0.67-0.97; P = .02).
Conclusions and relevance: The results of this cohort study suggest that PRO collection may be essential for trials because relying on the CTCAE to detect adverse events may miss important symptoms. Moreover, since physicians in this study systematically missed substantial symptoms in certain patients, including younger patients and Black individuals or those of Other race and ethnicity, improving symptom detection may be a targetable mechanism to reduce disparities.
Conflict of interest statement
Figures


Comment in
-
Underrecognition of Symptoms During Breast Radiotherapy.JAMA Oncol. 2022 Oct 1;8(10):1510. doi: 10.1001/jamaoncol.2022.3242. JAMA Oncol. 2022. PMID: 35951331 No abstract available.
-
Underrecognition of Symptoms During Breast Radiotherapy.JAMA Oncol. 2022 Oct 1;8(10):1509-1510. doi: 10.1001/jamaoncol.2022.3239. JAMA Oncol. 2022. PMID: 35951332 No abstract available.
-
Underrecognition of Symptoms During Breast Radiotherapy-Reply.JAMA Oncol. 2022 Oct 1;8(10):1510-1511. doi: 10.1001/jamaoncol.2022.3245. JAMA Oncol. 2022. PMID: 35951335 No abstract available.
References
-
- Gilbert A, Ziegler L, Martland M, et al. Systematic review of radiation therapy toxicity reporting in randomized controlled trials of rectal cancer: a comparison of patient-reported outcomes and clinician toxicity reporting. Int J Radiat Oncol Biol Phys. 2015;92(3):555-567. doi: 10.1016/j.ijrobp.2015.02.021 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

