Evaluation of Upper Airway Stimulation for Adolescents With Down Syndrome and Obstructive Sleep Apnea
- PMID: 35446411
- PMCID: PMC9026239
- DOI: 10.1001/jamaoto.2022.0455
Evaluation of Upper Airway Stimulation for Adolescents With Down Syndrome and Obstructive Sleep Apnea
Abstract
Importance: Patients with Down syndrome have a high incidence of persistent obstructive sleep apnea (OSA) and limited treatment options. Upper airway hypoglossal stimulation has been shown to be effective for adults with OSA but has not yet been evaluated for pediatric populations.
Objective: To evaluate the safety and effectiveness of upper airway stimulation for adolescent patients with Down syndrome and severe OSA.
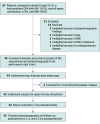
Design, setting, and participants: This prospective single-group multicenter cohort study with 1-year follow-up was conducted between April 1, 2015, and July 31, 2021, among a referred sample of 42 consecutive adolescent patients with Down syndrome and persistent severe OSA after adenotonsillectomy.
Intervention: Upper airway stimulation.
Main outcomes and measures: The prespecified primary outcomes were safety and the change in apnea-hypopnea index (AHI) from baseline to 12 months postoperatively. Polysomnographic and quality of life outcomes were assessed at 1, 2, 6, and 12 months postoperatively.
Results: Among the 42 patients (28 male patients [66.7%]; mean [SD] age, 15.1 [3.0] years), there was a mean (SD) decrease in AHI of 12.9 (13.2) events/h (95% CI, -17.0 to -8.7 events/h). With the use of a therapy response definition of a 50% decrease in AHI, the 12-month response rate was 65.9% (27 of 41), and 73.2% of patients (30 of 41) had a 12-month AHI of less than 10 events/h. The most common complication was temporary tongue or oral discomfort, which occurred in 5 patients (11.9%). The reoperation rate was 4.8% (n = 2). The mean (SD) improvement in the OSA-18 total score was 34.8 (20.3) (95% CI, -42.1 to -27.5), and the mean (SD) improvement in the Epworth Sleepiness Scale score was 5.1 (6.9) (95% CI, -7.4 to -2.8). The mean (SD) duration of nightly therapy was 9.0 (1.8) hours, with 40 patients (95.2%) using the device at least 4 hours a night.
Conclusions and relevance: Upper airway stimulation was able to be safely performed for 42 adolescents who had Down syndrome and persistent severe OSA after adenotonsillectomy with positive airway pressure intolerance. There was an acceptable adverse event profile with high rates of therapy response and quality of life improvement.
Trial registration: ClinicalTrials.gov Identifier: NCT02344108.
Conflict of interest statement
Figures
Comment in
-
Upper Airway Stimulation for Children With Down Syndrome and Obstructive Sleep Apnea-A New Frontier.JAMA Otolaryngol Head Neck Surg. 2022 Jun 1;148(6):529-530. doi: 10.1001/jamaoto.2022.0548. JAMA Otolaryngol Head Neck Surg. 2022. PMID: 35446405 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

