Pathophysiology and management of bleeding and thrombosis in patients with liver disease
- PMID: 35446468
- PMCID: PMC9540811
- DOI: 10.1111/ijlh.13856
Pathophysiology and management of bleeding and thrombosis in patients with liver disease
Abstract
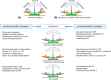
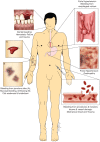
Patients with liver disease often develop complex changes in their haemostatic system. Frequently observed changes include thrombocytopaenia and altered plasma levels of most of the proteins involved in haemostasis. Although liver disease was historically classified as a haemostasis-related bleeding disorder, it has now been well established that the antihaemostatic changes that promote bleeding are compensated for by prohaemostatic changes. Conventional coagulation tests however do not accurately reflect these prohaemostatic changes, resulting in an underestimation of haemostatic potential. Novel coagulation tests, such as viscoelastic tests (VETs) and thrombin generation assays (TGAs) better reflect the net result of the haemostatic changes in patients with liver disease, and demonstrate a new, "rebalanced" haemostatic status. Although rebalanced, this haemostatic status is more fragile than in patients without liver disease. Patients with liver disease are therefore not only at risk of bleeding but also at risk of thrombosis. Notably, however, many haemostatic complications in liver disease are not related to the haemostatic failure. It is, therefore, crucial to identify the cause of the bleed or thrombotic complication in order to provide adequate treatment. In this paper, we will elaborate on the haemostatic changes that occur in liver disease, reflect on laboratory and clinical studies over the last few years, and explore the pathophysiologies of bleeding and thrombosis in this specific patient group.
Keywords: bleeding; coagulation; haemostasis; liver disease; thrombosis.
© 2022 The Authors. International Journal of Laboratory Hematology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Haemostatic alterations and management of haemostasis in patients with cirrhosis.J Hepatol. 2022 Jun;76(6):1291-1305. doi: 10.1016/j.jhep.2021.11.004. J Hepatol. 2022. PMID: 35589251 Review.
-
Unravelling the coagulation paradox in liver cirrhosis: challenges and insights.Acta Clin Belg. 2024 Dec;79(6):451-461. doi: 10.1080/17843286.2025.2469906. Epub 2025 Feb 21. Acta Clin Belg. 2024. PMID: 39981790 Review.
-
[Coagulation disorders in liver cirrhosis - Diagnostics and management].Dtsch Med Wochenschr. 2024 Aug;149(16):963-973. doi: 10.1055/a-2330-3564. Epub 2024 Aug 2. Dtsch Med Wochenschr. 2024. PMID: 39094601 Review. German.
-
Haemostasis and thrombosis in liver disease.Br J Haematol. 2010 Feb;148(4):507-21. doi: 10.1111/j.1365-2141.2009.08021.x. Epub 2009 Dec 8. Br J Haematol. 2010. PMID: 19995396 Review.
-
In vitro efficacy of pro- and anticoagulant strategies in compensated and acutely ill patients with cirrhosis.Liver Int. 2018 Nov;38(11):1988-1996. doi: 10.1111/liv.13882. Epub 2018 May 30. Liver Int. 2018. PMID: 29768734 Free PMC article.
Cited by
-
Direct oral anticoagulants in cirrhosis: Rationale and current evidence.JHEP Rep. 2024 May 9;6(8):101116. doi: 10.1016/j.jhepr.2024.101116. eCollection 2024 Aug. JHEP Rep. 2024. PMID: 39100819 Free PMC article. Review.
-
Cirrhosis Progression Is Not Associated with Clinically Significant Alterations in Global Hemostasis Assessed by Thromboelastography.J Clin Med. 2024 Nov 4;13(21):6614. doi: 10.3390/jcm13216614. J Clin Med. 2024. PMID: 39518754 Free PMC article.
-
Contemporary management of major haemorrhage in critical care.Intensive Care Med. 2024 Mar;50(3):319-331. doi: 10.1007/s00134-023-07303-5. Epub 2024 Jan 8. Intensive Care Med. 2024. PMID: 38189930 Review.
-
[Acute management of bleeding complications and coagulation disorders in critically ill patients with liver cirrhosis].Med Klin Intensivmed Notfmed. 2025 Feb 10. doi: 10.1007/s00063-024-01242-9. Online ahead of print. Med Klin Intensivmed Notfmed. 2025. PMID: 39928156 Review. German.
-
Treatment of portal vein thrombosis in cirrhosis with anticoagulation-more than meets the eye?Hepatobiliary Surg Nutr. 2024 Apr 3;13(2):321-324. doi: 10.21037/hbsn-23-669. Epub 2024 Mar 27. Hepatobiliary Surg Nutr. 2024. PMID: 38617493 Free PMC article. No abstract available.
References
-
- Northup PG, Garcia‐Pagan JC, Garcia‐Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73(1):366‐413. doi:10.1002/hep.31646 - DOI - PubMed
-
- Lisman T, Hernandez‐Gea V, Magnusson M, et al. The concept of rebalanced hemostasis in patients with liver disease: communication from the ISTH SSC working group on hemostatic management of patients with liver disease. J Thromb Haemost. 2021;19(4):1116‐1122. doi:10.1111/jth.15239 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

