A Folding-Based Electrochemical Aptasensor for the Single-Step Detection of the SARS-CoV-2 Spike Protein
- PMID: 35446532
- PMCID: PMC9045037
- DOI: 10.1021/acsami.2c02405
A Folding-Based Electrochemical Aptasensor for the Single-Step Detection of the SARS-CoV-2 Spike Protein
Abstract
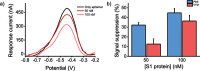
Efficient and timely testing has taken center stage in the management, control, and monitoring of the current COVID-19 pandemic. Simple, rapid, cost-effective diagnostics are needed that can complement current polymerase chain reaction-based methods and lateral flow immunoassays. Here, we report the development of an electrochemical sensing platform based on single-walled carbon nanotube screen-printed electrodes (SWCNT-SPEs) functionalized with a redox-tagged DNA aptamer that specifically binds to the receptor binding domain of the SARS-CoV-2 spike protein S1 subunit. Single-step, reagentless detection of the S1 protein is achieved through a binding-induced, concentration-dependent folding of the DNA aptamer that reduces the efficiency of the electron transfer process between the redox tag and the electrode surface and causes a suppression of the resulting amperometric signal. This aptasensor is specific for the target S1 protein with a dissociation constant (KD) value of 43 ± 4 nM and a limit of detection of 7 nM. We demonstrate that the target S1 protein can be detected both in a buffer solution and in an artificial viral transport medium widely used for the collection of nasopharyngeal swabs, and that no cross-reactivity is observed in the presence of different, non-target viral proteins. We expect that this SWCNT-SPE-based format of electrochemical aptasensor will prove useful for the detection of other protein targets for which nucleic acid aptamer ligands are made available.
Keywords: COVID-19; DNA aptamer; DNA nanotechnology; electrochemical sensors; single-walled carbon nanotubes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Label-free electrochemical aptasensor for rapid detection of SARS-CoV-2 spike glycoprotein based on the composite of Cu(OH)2 nanorods arrays as a high-performance surface substrate.Bioelectrochemistry. 2022 Aug;146:108106. doi: 10.1016/j.bioelechem.2022.108106. Epub 2022 Mar 23. Bioelectrochemistry. 2022. PMID: 35339949 Free PMC article.
-
Computational aptamer design for spike glycoprotein (S) (SARS CoV-2) detection with an electrochemical aptasensor.Appl Microbiol Biotechnol. 2024 Mar 12;108(1):259. doi: 10.1007/s00253-024-13066-w. Appl Microbiol Biotechnol. 2024. PMID: 38470514 Free PMC article.
-
Rapid and simple viral protein detection by functionalized 2D MoS2/graphene electrochemiluminescence aptasensor.Talanta. 2024 Aug 15;276:126293. doi: 10.1016/j.talanta.2024.126293. Epub 2024 May 20. Talanta. 2024. PMID: 38788383
-
Electrochemical biosensors for SARS-CoV-2 detection: Voltametric or impedimetric transduction?Bioelectrochemistry. 2022 Oct;147:108190. doi: 10.1016/j.bioelechem.2022.108190. Epub 2022 Jun 11. Bioelectrochemistry. 2022. PMID: 35738049 Free PMC article. Review.
-
Electrochemical SARS-CoV-2 Sensing at Point-of-Care and Artificial Intelligence for Intelligent COVID-19 Management.ACS Appl Bio Mater. 2020 Nov 16;3(11):7306-7325. doi: 10.1021/acsabm.0c01004. Epub 2020 Oct 27. ACS Appl Bio Mater. 2020. PMID: 35019473 Review.
Cited by
-
The Emergence of Carbon Nanomaterials as Effective Nano-Avenues to Fight against COVID-19.Materials (Basel). 2023 Jan 25;16(3):1068. doi: 10.3390/ma16031068. Materials (Basel). 2023. PMID: 36770075 Free PMC article. Review.
-
Aptamers and Nanobodies as New Bioprobes for SARS-CoV-2 Diagnostic and Therapeutic System Applications.Biosensors (Basel). 2024 Mar 15;14(3):146. doi: 10.3390/bios14030146. Biosensors (Basel). 2024. PMID: 38534253 Free PMC article. Review.
-
"Clickable" graphene nanoribbons for biosensor interfaces.Nanoscale Horiz. 2024 Mar 25;9(4):598-608. doi: 10.1039/d3nh00590a. Nanoscale Horiz. 2024. PMID: 38385442 Free PMC article.
-
Aptamers 101: aptamer discovery and in vitro applications in biosensors and separations.Chem Sci. 2023 May 2;14(19):4961-4978. doi: 10.1039/d3sc00439b. eCollection 2023 May 17. Chem Sci. 2023. PMID: 37206388 Free PMC article. Review.
-
Diagnostics and analysis of SARS-CoV-2: current status, recent advances, challenges and perspectives.Chem Sci. 2023 May 3;14(23):6149-6206. doi: 10.1039/d2sc06665c. eCollection 2023 Jun 14. Chem Sci. 2023. PMID: 37325147 Free PMC article. Review.
References
-
- Giannetto M.; Bianchi V.; Gentili S.; Fortunati S.; De Munari I.; Careri M. An Integrated IoT-Wi-Fi Board for Remote Data Acquisition and Sharing from Innovative Immunosensors. Case of Study: Diagnosis of Celiac Disease. Sens. Actuators, B 2018, 273, 1395–1403. 10.1016/j.snb.2018.07.056. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

