Diastereoselective Substitution Reactions of Acyclic β-Alkoxy Acetals via Electrostatically Stabilized Oxocarbenium Ion Intermediates
- PMID: 35446592
- PMCID: PMC9817112
- DOI: 10.1021/acs.orglett.2c01004
Diastereoselective Substitution Reactions of Acyclic β-Alkoxy Acetals via Electrostatically Stabilized Oxocarbenium Ion Intermediates
Abstract
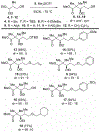
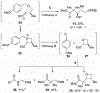
Substitution reactions of acyclic β-alkoxy acetals proceeded with generally high diastereoselectivities (>90:10) to form the anti product. Mechanistic experiments supplemented with computational studies suggest that, upon activation of the acetal, the resulting oxocarbenium ion is electrostatically stabilized by the β-alkoxy group. This stabilization defines the conformation of the reactive intermediate, which can be attacked preferentially from the more exposed face, leading to the observed products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
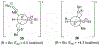

References
-
- Williams RJ; McGill NW; White JM; Williams SJ Neighboring Group Participation in Glycosylation Reactions by 2,6-Disubstituted 2-O-Benzoyl groups: A Mechanistic Investigation. J. Carbohydr. Chem 2010, 29, 236.
-
- Bérces A; Enright G; Nukada T; Whitfield DM The Conformational Origin of the Barrier to the Formation of Neighboring Group Assistance in Glycosylation Reactions: A Dynamical Density Functional Theory Study. J. Am. Chem. Soc 2001, 123, 5460. - PubMed
-
- Nukada T; Bérces A; Zgierski MZ; Whitfield DM Exploring the Mechanism of Neighboring Group Assisted Glycosylation Reactions. J. Am. Chem. Soc 1998, 120, 13291.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

