Stabilizing microbial communities by looped mass transfer
- PMID: 35446625
- PMCID: PMC9169928
- DOI: 10.1073/pnas.2117814119
Stabilizing microbial communities by looped mass transfer
Abstract
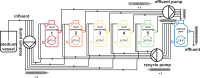
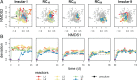
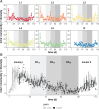
Building and changing a microbiome at will and maintaining it over hundreds of generations has so far proven challenging. Despite best efforts, complex microbiomes appear to be susceptible to large stochastic fluctuations. Current capabilities to assemble and control stable complex microbiomes are limited. Here, we propose a looped mass transfer design that stabilizes microbiomes over long periods of time. Five local microbiomes were continuously grown in parallel for over 114 generations and connected by a loop to a regional pool. Mass transfer rates were altered and microbiome dynamics were monitored using quantitative high-throughput flow cytometry and taxonomic sequencing of whole communities and sorted subcommunities. Increased mass transfer rates reduced local and temporal variation in microbiome assembly, did not affect functions, and overcame stochasticity, with all microbiomes exhibiting high constancy and increasing resistance. Mass transfer synchronized the structures of the five local microbiomes and nestedness of certain cell types was eminent. Mass transfer increased cell number and thus decreased net growth rates μ′. Subsets of cells that did not show net growth μ′SCx were rescued by the regional pool R and thus remained part of the microbiome. The loop in mass transfer ensured the survival of cells that would otherwise go extinct, even if they did not grow in all local microbiomes or grew more slowly than the actual dilution rate D would allow. The rescue effect, known from metacommunity theory, was the main stabilizing mechanism leading to synchrony and survival of subcommunities, despite differences in cell physiological properties, including growth rates.
Keywords: metacommunity assembly; microbial community cytometry; microbial ecology; single-cell analytics; stability.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Grimm V., Wissel C., Babel, or the ecological stability discussions: An inventory and analysis of terminology and a guide for avoiding confusion. Oecologia 109, 323–334 (1997). - PubMed
-
- Weise H., et al. , Resilience trinity: Safeguarding ecosystem functioning and services across three different time horizons and decision contexts. Oikos 129, 445–456 (2020).
-
- Angenent L. T., Magdalena J. A., Jeon B. S., Usack J. G., Eco-mimicry opens new doors for bioprocess engineers. Joule 4, 2074–2077 (2020).
-
- D’Hondt K., et al. , Microbiome innovations for a sustainable future. Nat. Microbiol. 6, 138–142 (2021). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

