Dynamics of Long-Term Patient-Reported Quality of Life and Health Behaviors After Adjuvant Breast Cancer Chemotherapy
- PMID: 35446677
- PMCID: PMC9509127
- DOI: 10.1200/JCO.21.00277
Dynamics of Long-Term Patient-Reported Quality of Life and Health Behaviors After Adjuvant Breast Cancer Chemotherapy
Abstract
Purpose: We aimed to characterize long-term quality of life (QOL) trajectories among patients with breast cancer treated with adjuvant chemotherapy and to identify related patterns of health behaviors.
Methods: Female stage I-III breast cancer patients receiving chemotherapy in CANTO (CANcer TOxicity; ClinicalTrials.gov identifier: NCT01993498) were included. Trajectories of QOL (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 Summary Score) and associations with trajectory group membership were identified by iterative estimations of group-based trajectory models and multivariable multinomial logistic regression, respectively.
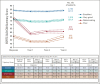
Results: Four trajectory groups were identified (N = 4,131): excellent (51.7%), very good (31.7%), deteriorating (10.0%), and poor (6.6%) QOL. The deteriorating trajectory group reported fairly good baseline QOL (mean [95% CI], 78.3/100 [76.2 to 80.5]), which significantly worsened at year-1 (58.1/100 [56.4 to 59.9]) and never recovered to pretreatment values through year-4 (61.1/100 [59.0 to 63.3]) postdiagnosis. Healthy behaviors were associated with better performing trajectory groups. Obesity (adjusted odds ratio [aOR] v lean, 1.51 [95% CI, 1.28 to 1.79]; P < .0001) and current smoking (aOR v never, 1.52 [95% CI, 1.27 to 1.82]; P < .0001) at diagnosis were associated with membership to the deteriorating group, which was also characterized by a higher prevalence of patients with excess body weight and insufficient physical activity through year-4 and by frequent exposure to tobacco smoking during chemotherapy. Additional factors associated with membership to the deteriorating group included younger age (aOR, 1-year decrement 1.01 [95% CI, 1.01 to 1.02]; P = .043), comorbidities (aOR v no, 1.22 [95% CI, 1.06 to 1.40]; P = .005), lower income (aOR v wealthier households, 1.21 [95% CI, 1.07 to 1.37]; P = .002), and endocrine therapy (aOR v no, 1.14 [95% CI, 1.01 to 1.30]; P = .047).
Conclusion: This latent-class analysis identified some patients with upfront poor QOL and a high-risk cluster with severe, persistent postchemotherapy QOL deterioration. Screening relevant patient-level characteristics may inform tailored interventions to mitigate the detrimental impact of chemotherapy and preserve QOL, including early addressal of behavioral concerns and provision of healthy lifestyle support programs.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

References
-
- Berry DA, Cronin KA, Plevritis SK, et al. : Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784-1792, 2005 - PubMed
-
- DeSantis CE, Bray F, Ferlay J, et al. : International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev 24:1495-1506, 2015 - PubMed
-
- SEER Cancer Statistics Review 1975-2006-Previous Version—SEER Cancer Statistics. https://seer.cancer.gov/archive/csr/1975_2006/

