Long-term persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein-specific and neutralizing antibodies in recovered COVID-19 patients
- PMID: 35446889
- PMCID: PMC9022880
- DOI: 10.1371/journal.pone.0267102
Long-term persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein-specific and neutralizing antibodies in recovered COVID-19 patients
Abstract
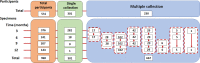
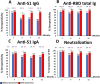
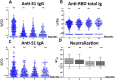
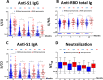
Understanding antibody responses after natural severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can guide the coronavirus disease 2019 (COVID-19) vaccine schedule, especially in resource-limited settings. This study aimed to assess the dynamics of SARS-CoV-2 antibodies, including anti-spike protein 1 (S1) immunoglobulin (Ig)G, anti-receptor-binding domain (RBD) total Ig, anti-S1 IgA, and neutralizing antibody against wild-type SARS-CoV-2 over time in a cohort of patients who were previously infected with the wild-type SARS-CoV-2. Between March and May 2020, 531 individuals with virologically confirmed cases of wild-type SARS-CoV-2 infection were enrolled in our immunological study. Blood samples were collected at 3-, 6-, 9-, and 12-months post symptom onset or detection of SARS-CoV-2 by RT-PCR (in asymptomatic individuals). The neutralizing titers against SARS-CoV-2 were detected in 95.2%, 86.7%, 85.0%, and 85.4% of recovered COVID-19 patients at 3, 6, 9, and 12 months after symptom onset, respectively. The seropositivity rate of anti-S1 IgG, anti-RBD total Ig, anti-S1 IgA, and neutralizing titers remained at 68.6%, 89.6%, 77.1%, and 85.4%, respectively, at 12 months after symptom onset. We observed a high level of correlation between neutralizing and SARS-CoV-2 spike protein-specific antibody titers. The half-life of neutralizing titers was estimated at 100.7 days (95% confidence interval = 44.5-327.4 days, R2 = 0.106). These results support that the decline in serum antibody levels over time in both participants with severe disease and mild disease were depended on the symptom severity, and the individuals with high IgG antibody titers experienced a significantly longer persistence of SARS-CoV-2-specific antibody responses than those with lower titers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Response and Duration of Serum Anti-SARS-CoV-2 Antibodies After Inactivated Vaccination Within 160 Days.Front Immunol. 2021 Dec 23;12:786554. doi: 10.3389/fimmu.2021.786554. eCollection 2021. Front Immunol. 2021. PMID: 35003104 Free PMC article.
-
Kinetics of SARS-CoV-2 Specific and Neutralizing Antibodies over Seven Months after Symptom Onset in COVID-19 Patients.Microbiol Spectr. 2021 Oct 31;9(2):e0059021. doi: 10.1128/Spectrum.00590-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34550000 Free PMC article.
-
Are SARS-CoV-2 Antibodies Detectable in Human Milk After Vaccination Against COVID-19?J Pediatric Infect Dis Soc. 2022 Apr 30;11(4):126. doi: 10.1093/jpids/piac024. J Pediatric Infect Dis Soc. 2022. PMID: 35394545 Free PMC article.
-
SARS-CoV-2 spike S2-specific neutralizing antibodies.Emerg Microbes Infect. 2023 Dec;12(2):2220582. doi: 10.1080/22221751.2023.2220582. Emerg Microbes Infect. 2023. PMID: 37254830 Free PMC article. Review.
-
Long-Term Immunity and Antibody Response: Challenges for Developing Efficient COVID-19 Vaccines.Antibodies (Basel). 2022 May 12;11(2):35. doi: 10.3390/antib11020035. Antibodies (Basel). 2022. PMID: 35645208 Free PMC article. Review.
Cited by
-
Persistent humoral and CD4+ TH cell immunity after mild SARS-COV-2 infection-The CoNAN long-term study.Front Immunol. 2023 Jan 13;13:1095129. doi: 10.3389/fimmu.2022.1095129. eCollection 2022. Front Immunol. 2023. PMID: 36713390 Free PMC article.
-
An update on lateral flow immunoassay for the rapid detection of SARS-CoV-2 antibodies.AIMS Microbiol. 2023 Apr 13;9(2):375-401. doi: 10.3934/microbiol.2023020. eCollection 2023. AIMS Microbiol. 2023. PMID: 37091823 Free PMC article. Review.
-
The risk factors of SARS-CoV-2 antibody level differences in healthcare workers post vaccination in Siloam hospitals: A nationwide multicenter study.Infect Med (Beijing). 2022 Dec;1(4):229-235. doi: 10.1016/j.imj.2022.10.001. Epub 2022 Oct 20. Infect Med (Beijing). 2022. PMID: 38013914 Free PMC article.
-
Design and performance characteristics of the Elecsys anti-SARS-CoV-2 S assay.Front Immunol. 2022 Dec 2;13:1002576. doi: 10.3389/fimmu.2022.1002576. eCollection 2022. Front Immunol. 2022. PMID: 36532081 Free PMC article.
-
Longitudinal monitoring of SARS-CoV-2 spike protein-specific antibody responses in Lower Austria.PLoS One. 2022 Jul 27;17(7):e0271382. doi: 10.1371/journal.pone.0271382. eCollection 2022. PLoS One. 2022. PMID: 35895668 Free PMC article.
References
-
- To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al.. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–74. Epub 2020/03/28. doi: 10.1016/S1473-3099(20)30196-1 ; PubMed Central PMCID: PMC7158907. - DOI - PMC - PubMed
-
- Brouwer PJM, Caniels TG, van der Straten K, Snitselaar JL, Aldon Y, Bangaru S, et al.. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643–50. Epub 2020/06/17. doi: 10.1126/science.abc5902 ; PubMed Central PMCID: PMC7299281. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

