Smallpox vaccination induces a substantial increase in commensal skin bacteria that promote pathology and influence the host response
- PMID: 35446919
- PMCID: PMC9022886
- DOI: 10.1371/journal.ppat.1009854
Smallpox vaccination induces a substantial increase in commensal skin bacteria that promote pathology and influence the host response
Abstract
Interactions between pathogens, host microbiota and the immune system influence many physiological and pathological processes. In the 20th century, widespread dermal vaccination with vaccinia virus (VACV) led to the eradication of smallpox but how VACV interacts with the microbiota and whether this influences the efficacy of vaccination are largely unknown. Here we report that intradermal vaccination with VACV induces a large increase in the number of commensal bacteria in infected tissue, which enhance recruitment of inflammatory cells, promote tissue damage and influence the host response. Treatment of vaccinated specific-pathogen-free (SPF) mice with antibiotic, or infection of genetically-matched germ-free (GF) animals caused smaller lesions without alteration in virus titre. Tissue damage correlated with enhanced neutrophil and T cell infiltration and levels of pro-inflammatory tissue cytokines and chemokines. One month after vaccination, GF and both groups of SPF mice had equal numbers of VACV-specific CD8+ T cells and were protected from disease induced by VACV challenge, despite lower levels of VACV-neutralising antibodies observed in GF animals. Thus, skin microbiota may provide an adjuvant-like stimulus during vaccination with VACV and influence the host response to vaccination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
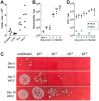


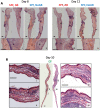

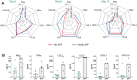

Similar articles
-
Capturing the natural diversity of the human antibody response against vaccinia virus.J Virol. 2011 Feb;85(4):1820-33. doi: 10.1128/JVI.02127-10. Epub 2010 Dec 8. J Virol. 2011. PMID: 21147924 Free PMC article.
-
Adverse events post smallpox-vaccination: insights from tail scarification infection in mice with Vaccinia virus.PLoS One. 2011 Apr 15;6(4):e18924. doi: 10.1371/journal.pone.0018924. PLoS One. 2011. PMID: 21526210 Free PMC article.
-
Smallpox vaccine, ACAM2000: Sites and duration of viral shedding and effect of povidone iodine on scarification site shedding and immune response.Vaccine. 2015 Jun 12;33(26):2990-6. doi: 10.1016/j.vaccine.2015.04.062. Epub 2015 Apr 27. Vaccine. 2015. PMID: 25930115 Clinical Trial.
-
Vaccinia Virus Natural Infections in Brazil: The Good, the Bad, and the Ugly.Viruses. 2017 Nov 15;9(11):340. doi: 10.3390/v9110340. Viruses. 2017. PMID: 29140260 Free PMC article. Review.
-
A vaccinia virus renaissance: new vaccine and immunotherapeutic uses after smallpox eradication.Hum Vaccin Immunother. 2012 Jul;8(7):961-70. doi: 10.4161/hv.21080. Epub 2012 Jul 1. Hum Vaccin Immunother. 2012. PMID: 22777090 Free PMC article. Review.
Cited by
-
IL-10 inhibition during immunization improves vaccine-induced protection against Staphylococcus aureus infection.JCI Insight. 2024 May 28;9(13):e178216. doi: 10.1172/jci.insight.178216. JCI Insight. 2024. PMID: 38973612 Free PMC article.
-
Comparison of the Effectiveness of Transepidemal and Intradermal Immunization of Mice with the Vacinia Virus.Acta Naturae. 2022 Oct-Dec;14(4):111-118. doi: 10.32607/actanaturae.11857. Acta Naturae. 2022. PMID: 36694907 Free PMC article.
-
Enhanced Effects of ISA 207 Adjuvant via Intradermal Route in Foot-and-Mouth Disease Vaccine for Pigs.Vaccines (Basel). 2024 Aug 26;12(9):963. doi: 10.3390/vaccines12090963. Vaccines (Basel). 2024. PMID: 39339996 Free PMC article.
-
Suppression of innate immunity by the vaccinia virus protein N1 promotes skin microbiota expansion and increased immune infiltration following vaccination.J Gen Virol. 2022 Nov;103(11):10.1099/jgv.0.001814. doi: 10.1099/jgv.0.001814. J Gen Virol. 2022. PMID: 36748513 Free PMC article.
-
Interferon lambda restricts herpes simplex virus skin disease by suppressing neutrophil-mediated pathology.mBio. 2024 Apr 10;15(4):e0262323. doi: 10.1128/mbio.02623-23. Epub 2024 Mar 1. mBio. 2024. PMID: 38426749 Free PMC article.
References
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva1988.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

