Efficacy of combined immunosuppression with or without eltrombopag in children with newly diagnosed aplastic anemia
- PMID: 35446936
- PMCID: PMC10027512
- DOI: 10.1182/bloodadvances.2021006716
Efficacy of combined immunosuppression with or without eltrombopag in children with newly diagnosed aplastic anemia
Abstract
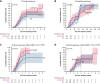
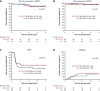
We compared the efficacy and safety of eltrombopag (ELTR) combined with immunosuppressive therapy (IST) and IST alone in treatment-naïve children with severe (SAA) and very severe (vSAA) aplastic anemia. Ninety-eight pediatric patients were randomized to receive horse antithymocyte globulin (hATG) and cyclosporin A (CsA) with (n = 49) or without (n = 49) ELTR. The primary endpoint was the overall response rate (ORR) at 4 months. After 4 months, nonresponders were crossed over to the alternative group. In all patients, the ORR in ELTR + IST and IST groups was similar (65% vs 53%; P = .218); however, the complete response (CR) rate was significantly higher in the ELTR + IST group (31% vs 12%; P = .027). In severity subgroups, the ORR was 89% vs 57% (P = .028) in favor of IST + ELTR in SAA, but it did not differ in patients with vSAA (52% vs 50%; P = .902). At 6 months after the crossover, 61% of initial ELTR(-) patients achieved a response compared with 17% of initial ELTR(+) patients (P = .016). No significant difference in ELTR + IST and IST groups was observed in the 3-year overall survival (OS) (89% vs 91%; P = .673) or the 3-year event-free survival (EFS) (53% vs 41%; P = .326). There was no unexpected toxicity related to ELTR. Adding ELTR to standard IST was well tolerated and increased the CR rate. The greatest benefit from ELTR combined with IST was observed in patients with SAA but not in those with vSAA. The second course of IST resulted in a high ORR in initial ELTR(-) patients who added ELTR and had limited efficacy among patients who received ELTR upfront. This trial was registered at Clinicaltrials.gov as #NCT03413306.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.M. received lecturer’s fees from Novartis. M.M. received lecturer’s fees from Miltenyi Biotec. The remaining authors declare no competing financial interests.
Figures

References
-
- Dufour C, Pillon M, Sociè G, et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant. Br J Haematol. 2015;169(4):565–573. - PubMed
-
- Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129(11):1428–1436. - PubMed

