Impact of previous exposure to SARS-CoV-2 and of S-Trimer (SCB-2019) COVID-19 vaccination on the risk of reinfection: a randomised, double-blinded, placebo-controlled, phase 2 and 3 trial
- PMID: 35447085
- PMCID: PMC9015644
- DOI: 10.1016/S1473-3099(22)00144-X
Impact of previous exposure to SARS-CoV-2 and of S-Trimer (SCB-2019) COVID-19 vaccination on the risk of reinfection: a randomised, double-blinded, placebo-controlled, phase 2 and 3 trial
Abstract
Background: We previously reported the efficacy of the adjuvanted-protein COVID-19 vaccine candidate S-Trimer (SCB-2019) in adults who showed no evidence of previous exposure to SARS-CoV-2. In this study, we aimed to investigate the extent of protection afforded by previous exposure to SARS-CoV-2 on subsequent COVID-19 infection, as well as the efficacy, safety, and reactogenicity of SCB-2019 in participants who were enrolled in the Study evaluating Protective-Efficacy and safety of Clover's Trimeric Recombinant protein-based and Adjuvanted COVID-19 vaccine (SPECTRA) trial who had already been exposed to SARS-CoV-2 before vaccination.
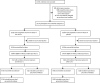
Methods: In a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial (SPECTRA) done at 31 sites in five countries, participants were randomly assigned 1:1 using the Cenduit Interactive Response Technology system (IQVIA, Durham, NC, USA), with a block size of six, to receive two doses of either SCB-2019 or placebo 21 days apart. The primary outcomes of the SPECTRA trial were vaccine efficacy, measured by real-time PCR (rtPCR)-confirmed COVID-19 of any severity, with onset from 14 days after the second vaccine dose, as well as the safety and solicited local and systemic adverse events in the phase 2 subset. Here, we present secondary analyses to calculate the protective efficacy due to previous exposure to SARS-CoV-2 against reinfection with COVID-19 according to severity in SPECTRA participants who had evidence of exposure to SARS-CoV-2 at baseline, including efficacy against identified viral variants, as well as efficacy of SCB-2019 vaccination in this population.
Findings: We enrolled 30 174 participants between March 24, 2021, and Aug 10, 2021. In the 14 670 participants who were randomly assigned to receive placebo, there were 418 (2·8%) confirmed cases of COVID-19; 65 (0·9%) of 7339 SARS-CoV-2-exposed participants, and 353 (4·8%) of 7331 SARS-CoV-2-naive participants (attack rates of 5·5 cases per 100 person-years for SARS-CoV-2-exposed participants and 32·4 cases per 100 person-years for SARS-CoV-2-naive participants). Protective efficacy due to previous exposure to SARS-CoV-2 was 83·2% (95% CI 78·0-87·3) against any COVID-19, 92·5% (82·9-97·3) against moderate-to-severe COVID-19, and 100% (59·3-100) against severe COVID-19; no SARS-CoV-2-exposed participants had hospitalisation associated with COVID-19. Protective efficacy against variants were 100% for alpha (B.1.1.7) and lambda (C.37) variants, 88·6% (14·9-99·7) for B.1.623, 93·6% (80·1-98·7) for gamma (P.1), and 92·4% (81·2-97·6) for mu (B.1.621) variants, and lowest against beta (B.1.351; 72·2% [33·1-89·9]) and delta (B.1.617.2; 77·2% [61·3-87·2]) variants. In addition, one dose of SCB-2019 had 49·9% (1·5-75·6) efficacy against any symptomatic COVID-19, and two doses had 64·2% (26·5-83·8) efficacy. SCB-2019 was well tolerated in SARS-CoV-2-exposed participants, but was associated with higher rates of injection site pain (89 [33·8%] of 263 participants) than placebo (16 [6·7%] of 239 participants). Rates of solicited systemic adverse events, severe adverse events, and serious adverse events were similar between vaccine and placebo groups, and with rates in SARS-CoV-2-naive vaccine recipients.
Interpretation: Previous exposure to SARS-CoV-2 decreased the risk and severity of subsequent COVID-19 infection, even against newly emerging variants. Protection is further enhanced by one or two doses of SCB-2019.
Funding: Clover Biopharmaceuticals, The Coalition for Epidemic Preparedness Innovations (CEPI).
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests IS, HHH, PL, CB, CV, and JL are full-time employees of Clover Biopharmaceuticals. FR is a statistical adviser, and SACC, RC, DA, PR, and GS are scientific advisers for Clover Biopharmaceuticals.
Figures

Comment in
-
The S-Trimer (SCB-2019) COVID-19 vaccine and reinfection with SARS-CoV-2.Lancet Infect Dis. 2022 Jul;22(7):916-917. doi: 10.1016/S1473-3099(22)00162-1. Epub 2022 Apr 18. Lancet Infect Dis. 2022. PMID: 35447084 Free PMC article. No abstract available.
References
-
- Our World in Data Coronavirus (COVID-19) vaccinations. Dec 16, 2021. https://ourworldindata.org/covid-vaccinations
-
- WHO Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
-
- WHO Update on Omicron. Nov 28, 2021. https://www.who.int/news/item/28-11-2021-update-on-omicron
-
- WHO COVID-19 vaccine tracker and landscape. Nov 16, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...
Uncited References
-
- Washington State Department of Health SARS-CoV-2 vaccine breakthrough surveillance and case information resource. Nov 24, 2021. https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/data-tables/...
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

