A mechanochemical model of the forward/backward movement of motor protein kinesin-1
- PMID: 35447112
- PMCID: PMC9117889
- DOI: 10.1016/j.jbc.2022.101948
A mechanochemical model of the forward/backward movement of motor protein kinesin-1
Abstract
Kinesin-1 is an ATP-driven, two-headed motor protein that transports intracellular cargoes (loads) along microtubules. The movement of kinesin-1 has generally been modeled according to its correlation with ATP cleavage (forward movement), synthesis (backward movement), or unproductive cleavage (futile consumption). Based on recent experimental observations, we formulate a mechanochemical model for this movement in which the forward/backward/futile cycle can be realized through multiple biochemical pathways. Our results show that the backward motion of kinesin-1 occurs mainly through backward sliding along the microtubule and is usually also coupled with ATP hydrolysis. We also found that with a low external load, about 80% of ATP is wasted (futile consumption) by kinesin-1. Furthermore, at high ATP concentrations or under high external loads, both heads of kinesin-1 are always in the ATP- or ADP ⋅ Pi-binding state and tightly bound to the microtubule, while at low ATP concentrations and low loads, kinesin-1 is mainly in the one-head-bound state. Unless the external load is near the stall force, the motion of kinesin-1 is almost deterministic.
Keywords: Biochemical pathways; Effective diffusion constant; Mean run length; Mean run time; Mean velocity.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
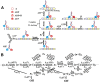

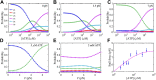


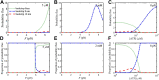
References
-
- Howard J. Sinauer Associates; Sunderland, MA: 2001. Mechanics of Motor Proteins and the Cytoskeleton.
-
- Vale R.D. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467. - PubMed
-
- Schliwa M. Wiley-Vch; Weinheim: 2003. Molecular Motors.
-
- Kolomeisky A.B. CRC Press, Taylor & Francis Group; Boca Raton, FL: 2015. Motor Proteins and Molecular Motors.
-
- Case R.B., Rice S., Hart C.L., Ly B., Vale R.D. Role of the kinesin neck linker and catalytic core in microtubulebased motility. Curr. Biol. 2000;380:157. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

