Induction of glutathione biosynthesis by glycine-based treatment mitigates atherosclerosis
- PMID: 35447412
- PMCID: PMC9044008
- DOI: 10.1016/j.redox.2022.102313
Induction of glutathione biosynthesis by glycine-based treatment mitigates atherosclerosis
Abstract
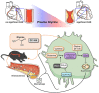
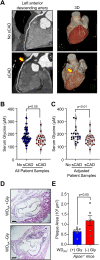
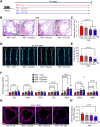
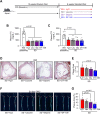
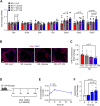
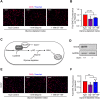
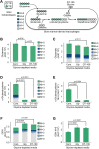
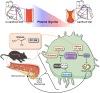
Lower circulating levels of glycine are consistently reported in association with cardiovascular disease (CVD), but the causative role and therapeutic potential of glycine in atherosclerosis, the underlying cause of most CVDs, remain to be established. Here, following the identification of reduced circulating glycine in patients with significant coronary artery disease (sCAD), we investigated a causative role of glycine in atherosclerosis by modulating glycine availability in atheroprone mice. We further evaluated the atheroprotective potential of DT-109, a recently identified glycine-based compound with dual lipid/glucose-lowering properties. Glycine deficiency enhanced, while glycine supplementation attenuated, atherosclerosis development in apolipoprotein E-deficient (Apoe-/-) mice. DT-109 treatment showed the most significant atheroprotective effects and lowered atherosclerosis in the whole aortic tree and aortic sinus concomitant with reduced superoxide. In Apoe-/- mice with established atherosclerosis, DT-109 treatment significantly reduced atherosclerosis and aortic superoxide independent of lipid-lowering effects. Targeted metabolomics and kinetics studies revealed that DT-109 induces glutathione formation in mononuclear cells. In bone marrow-derived macrophages (BMDMs), glycine and DT-109 attenuated superoxide formation induced by glycine deficiency. This was abolished in BMDMs from glutamate-cysteine ligase modifier subunit-deficient (Gclm-/-) mice in which glutathione biosynthesis is impaired. Metabolic flux and carbon tracing experiments revealed that glycine deficiency inhibits glutathione formation in BMDMs while glycine-based treatment induces de novo glutathione biosynthesis. Through a combination of studies in patients with CAD, in vivo studies using atherosclerotic mice and in vitro studies using macrophages, we demonstrated a causative role of glycine in atherosclerosis and identified glycine-based treatment as an approach to mitigate atherosclerosis through antioxidant effects mediated by induction of glutathione biosynthesis.
Keywords: Amino acids; Atherosclerosis; Glutathione; Glycine; Macrophages.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
O. Rom, Y. Zhao, J. Zhang, and Y.E. Chen have filed a patent application based on this work (Tri-peptides and treatment of metabolic, cardiovascular, and inflammatory disorders: PCT/US2019/046052). Y.E. Chen is the founder of Diapin Therapeutics, which provided DT-109 for this study. All other authors declare that they have no competing interests.
Figures
References
-
- Meléndez-Hevia E., De Paz-Lugo P., Cornish-Bowden A., Cárdenas M.L. A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J. Biosci. 2009;34:853–872. - PubMed
-
- Wang W., Wu Z., Dai Z., Yang Y., Wang J., Wu G. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids. 2013;45:463–477. - PubMed
-
- Hitzel J., Lee E., Zhang Y., Bibli S.I., Li X., Zukunft S., Pflüger B., Hu J., Schürmann C., Vasconez A.E., Oo J.A., Kratzer A., Kumar S., Rezende F., Josipovic I., Thomas D., Giral H., Schreiber Y., Geisslinger G., Fork C., Yang X., Sigala F., Romanoski C.E., Kroll J., Jo H., Landmesser U., Lusis A.J., Namgaladze D., Fleming I., Leisegang M.S., Zhu J., Brandes R.P. Oxidized phospholipids regulate amino acid metabolism through MTHFD2 to facilitate nucleotide release in endothelial cells. Nat. Commun. 2018;9:2292. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 HL150233/HL/NHLBI NIH HHS/United States
- K99 HL150233/HL/NHLBI NIH HHS/United States
- R01 HL147527/HL/NHLBI NIH HHS/United States
- R00 HL145131/HL/NHLBI NIH HHS/United States
- P20 GM121307/GM/NIGMS NIH HHS/United States
- R01 HL134569/HL/NHLBI NIH HHS/United States
- R01 HL153710/HL/NHLBI NIH HHS/United States
- R01 HL138139/HL/NHLBI NIH HHS/United States
- R01 HL137214/HL/NHLBI NIH HHS/United States
- R01 HL109946/HL/NHLBI NIH HHS/United States
- R01 HL149264/HL/NHLBI NIH HHS/United States
- R01 HL139755/HL/NHLBI NIH HHS/United States
- U2C DK110768/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

