Measurements of SARS-CoV-2 antibody dissociation rate constant by chaotrope-free biolayer interferometry in serum of COVID-19 convalescent patients
- PMID: 35447596
- PMCID: PMC8993703
- DOI: 10.1016/j.bios.2022.114237
Measurements of SARS-CoV-2 antibody dissociation rate constant by chaotrope-free biolayer interferometry in serum of COVID-19 convalescent patients
Abstract
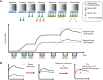
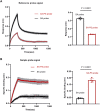
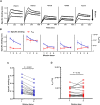
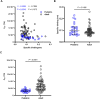
Kinetics measurements of antigen-antibody binding interactions are critical to understanding the functional efficiency of SARS-CoV-2 antibodies. Previously reported chaotrope-based avidity assays that rely on artificial disruption of binding do not reflect the natural binding kinetics. This study developed a chaotrope- and label-free biolayer interferometry (BLI) assay for the real-time monitoring of receptor binding domain (RBD) binding kinetics with SARS-CoV-2 spike protein in convalescent COVID-19 patients. An improved conjugation biosensor probe coated with streptavidin-polysaccharide (SA-PS) led to a six-fold increase of signal intensities and two-fold reduction of non-specific binding (NSB) compared to streptavidin only probe. Furthermore, by utilizing a separate reference probe and biotin-human serum albumin (B-HSA) blocking process to subtracted NSB signal in serum, this BLI biosensor can measure a wide range of the dissociation rate constant (koff), which can be measured without knowledge of the specific antibody concentrations. The clinical utility of this improved BLI kinetics assay was demonstrated by analyzing the koff values in sera of 24 pediatric (≤18 years old) and 63 adult (>18 years old) COVID-19 convalescent patients. Lower koff values for SARS-CoV-2 serum antibodies binding to RBD were measured in samples from children. This rapid, easy to operate and chaotrope-free BLI assay is suitable for clinical use and can be readily adapted to characterize SARS-CoV-2 antibodies developed by COVID-19 patients and vaccines.
Keywords: Avidity; Biolayer interferometry; COVID-19; Dissociation rate constant; Kinetics; SARS-CoV-2.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Auer S., Koho T., Uusi-Kerttula H., Vesikari T., Blazevic V., Hytönen V.P. Rapid and sensitive detection of norovirus antibodies in human serum with a biolayer interferometry biosensor. Sensor. Actuator. B Chem. 2015;221:507–514.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

